BRAF mutations (mainly V600E) are seen in many different tumors (see table below), and the presence of such mutations have therapeutic predictive and diagnostic information for the patient based on the type of tumor implicated. For example, BRAF mutated colorectal carcinomas, like KRAS mutated tumors do not respond to EGFR inhibitors, but BRAF mutated melanomas may respond to the BRAF kinase inhibitor, vemurafenib.
BRAF mutations can be detected by multiple different modalities with the most popular being Sanger sequencing and allele specific PCR. FISH is not helpful in identifying point mutations. More recently immunohistochemistry stains for the BRAF V600E mutation have been developed. The two primary available commercial clones are VE1 [Spring Bioscience, Pleasanton, CA] and anti-B-Raf mouse monoclonal antibody, 1:1000 [New East Biosciences, Malvern, PA]. In a comparison study by Routhier, et. al., they examined 152 tumors (multiple types), and found the VE1 clone to be 98% sensitive and 97% specific with a concordance rate to mutational analysis of 97%. The anti-B-Raf antibody did not perform as well with 95% sensitivity, 83% specificity, and a concordance rate with mutational analysis of 88%.
The excellent performance of the VE1 clone allows for the possibility of rapid low-cost screening for the BRAF V600E mutation.
Stain Interpretation
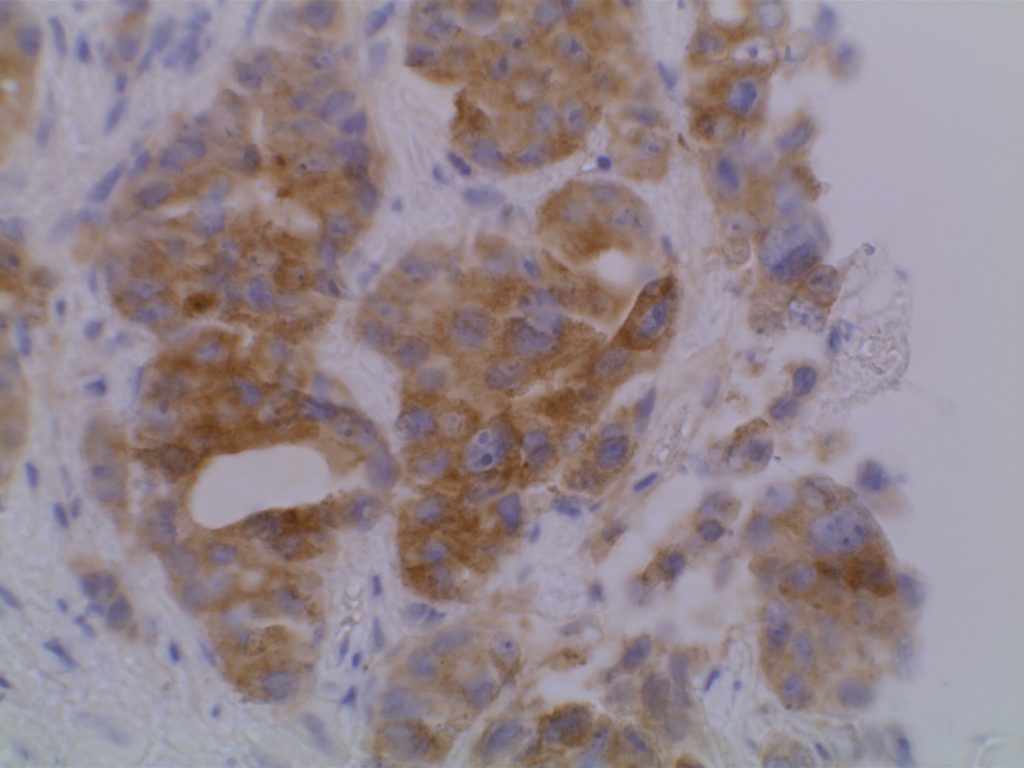
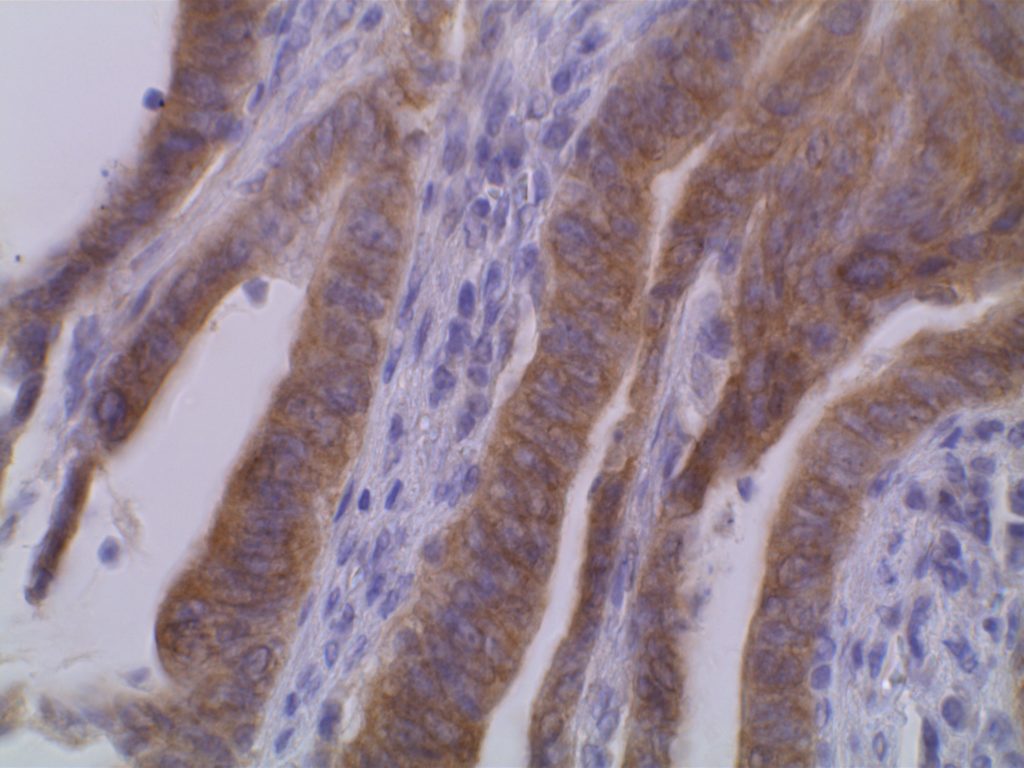
The VE1 clone was developed to detect the BRAF V600E mutated protein, and the IHC staining pattern is cytoplasmic. Expression pattern is usually diffuse, and the intensity can range from weak to strong. In a subset of cases, expression heterogeneity has been seen. This has raised the possibility of BRAF mutational heterogeneity within the tumor. More study is needed to further correlate IHC expression intensity and pattern expression with outcomes and response to therapy.
BRAF mutation incidence for specific tumor types, and clinical significance (Routhier, et. al.)
|
Tumor Type
|
Incidence
|
Clinical Significance
|
|
100%
|
Potential response to BRAF inhibitor therapy
|
|
|
Pleomorphic
Xanthoastrocytoma
|
66%
|
Potential response to BRAF inhibitor therapy
|
|
Malignant Melanoma
|
40-60%
|
May be responsive to BRAF inhibitor, vemurafenib
|
|
Papillary Thyroid Ca.
|
45%
|
Diagnostic confirmation, may indicate a more aggressive course
|
|
Ovarian Boarderline
Tumors
|
13%
|
|
|
Biliary Tract Ca.
|
20%
|
|
|
Colorectal Ca.
|
10%
(5-25%)
|
EGFR inhibitor resistance similar to KRAS mutated tumors; may also be used to separate out sporadic type MSI-H mutated tumors (~50-66% BRAF mutated)
|
|
Lung Adenoca.
|
<5%
|
Potential response to BRAF inhibitor therapy
|
Pitfalls
BRAF (VE1 clone) IHC expression has been detected in the pituitary gland (particularly ACTH secreting cells) and adrenal cortical tissue (strongest expression in the inner zone fasciculata) without detectable BRAF V600E mutations. (Mordes, et. al.) These factors should be considered when evaluating the specificity of this stain/clone.
Photomicrographs
References
Routhier, C. A., Mochel, M. C., Lynch, K., Dias-Santagata, D., Louis, D. N., & Hoang, M. P. (2013). Comparison of 2 monoclonal antibodies for immunohistochemical detection of BRAF V600E mutation in malignant melanoma, pulmonary carcinoma, gastrointestinal carcinoma, thyroid carcinoma, and gliomas. Human Pathology, 44(11), 2563–2570. doi:10.1016/j.humpath.2013.06.018
Busam, K. J., Hedvat, C., Pulitzer, M., Deimling, von, A., & Jungbluth, A. A. (2013). Immunohistochemical analysis of BRAF(V600E) expression of primary and metastatic melanoma and comparison with mutation status and melanocyte differentiation antigens of metastatic lesions. The American Journal of Surgical Pathology, 37(3), 413–420. doi:10.1097/PAS.0b013e318271249e
Toon, C. W., Walsh, M. D., Chou, A., Capper, D., Clarkson, A., Sioson, L., et al. (2013). BRAFV600E immunohistochemistry facilitates universal screening of colorectal cancers for Lynch syndrome. The American Journal of Surgical Pathology, 37(10), 1592–1602. doi:10.1097/PAS.0b013e31828f233d
Long, G. V., Wilmott, J. S., Capper, D., Preusser, M., Zhang, Y. E., Thompson, J. F., et al. (2013). Immunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanoma. The American Journal of Surgical Pathology, 37(1), 61–65. doi:10.1097/PAS.0b013e31826485c0
Jin, M., Hampel, H., Zhou, X., Schunemann, L., Yearsley, M., & Frankel, W. L. (2013). BRAF V600E mutation analysis simplifies the testing algorithm for Lynch syndrome. American Journal of Clinical Pathology, 140(2), 177–183. doi:10.1309/AJCPB9FOVH1HGKFR
Geiersbach, K. B., & Samowitz, W. S. (2011). Microsatellite instability and colorectal cancer. Archives of Pathology & Laboratory Medicine, 135(10), 1269–1277. doi:10.5858/arpa.2011-0035-RA
Mordes, D. A., Lynch, K., Campbell, S., Dias-Santagata, D., Nose, V., Louis, D. N., & Hoang, M. P. (2014). VE1 Antibody Immunoreactivity in Normal Anterior Pituitary and Adrenal Cortex Without Detectable BRAF V600E Mutations. American Journal of Clinical Pathology, 141(6), 811–815. doi:10.1309/AJCP37TLZLTUAOJL