Summary (Breast Carcinoma)
- Nuclear Marker
- Stain is reported as PERCENT STAINING OF TUMOR CELLS and STAIN INTENSITY (1+, 2+, 3+)
- 1% or greater nuclear expression in tumor cells is considered positive, and therefore eligible to receive hormonal therapy.
- CAP-ASCO recommendations are for <1 hr. from time of excision/biopsy to having a cut edge of tumor in 10% neutral bufferedormalin fixative. Fixation window of 6-72 hrs. These times should be noted in the pathology report (time of excision, time in gross room, and time in fixative).
- Negative staining results in biopsy material without an internal control should be repeated on the excisional specimen using blocks with both tumor and benign breast parenchyma.
General
Estrogen Receptor (ER) is a nuclear marker, which is most commonly used to identify breast carcinomas that may be responsive hormonal therapy (e.g. Tamoxifen). It also conveys prognostic information (ER+ has a more favorable prognosis). In normal breast duct epithelium ER will show variable patchy expression, but does not normally stain myoepithelial cells.
Interpretation
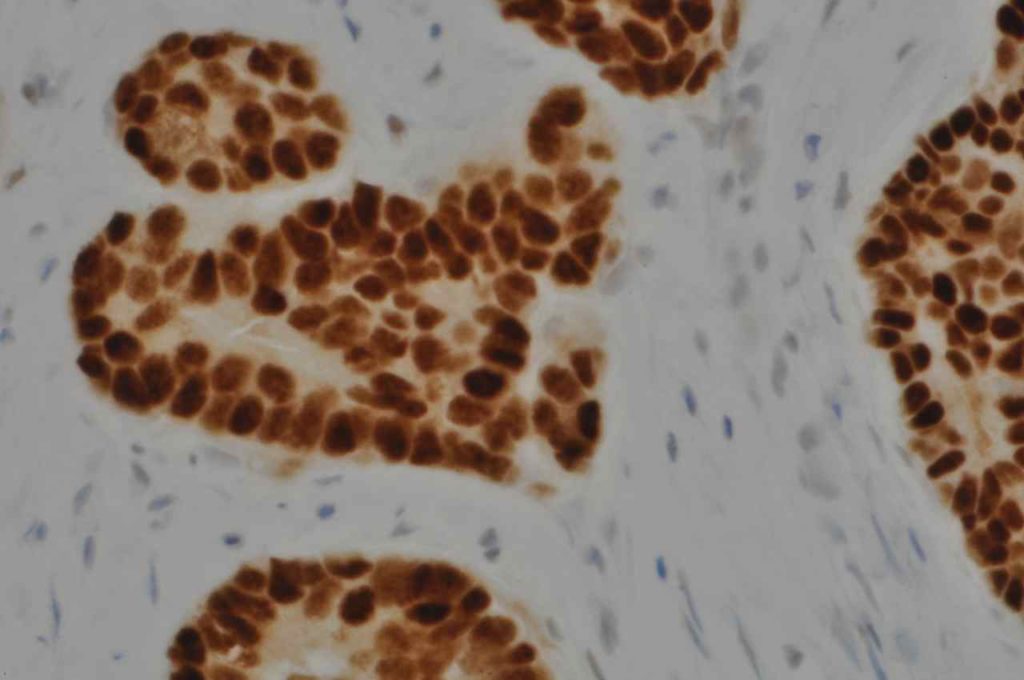
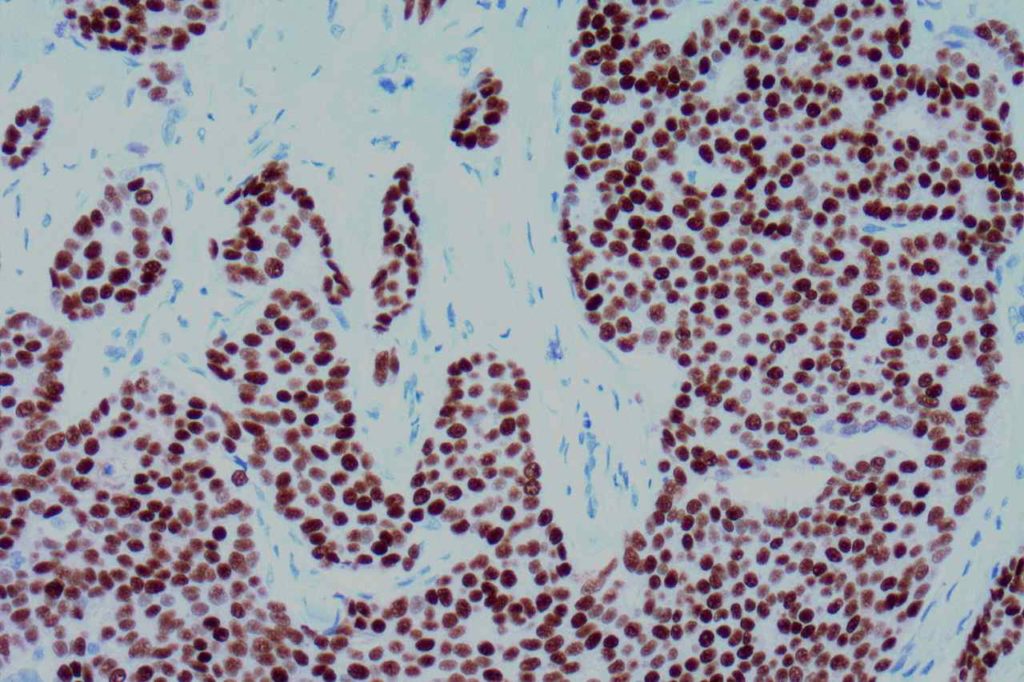
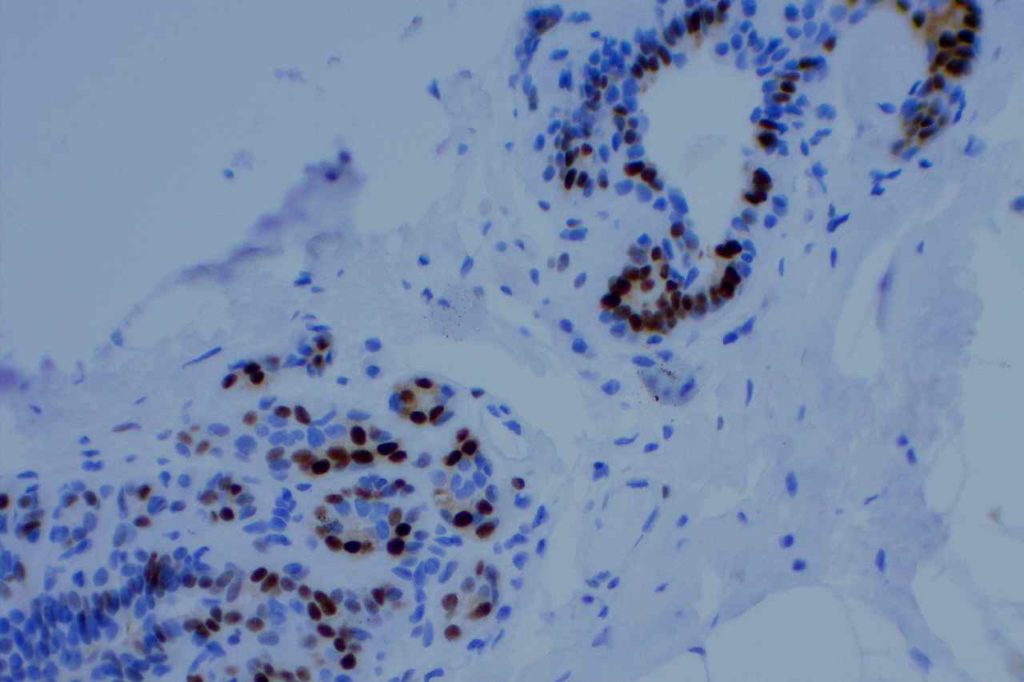
Interpretation of ER expression in breast carcinoma is based on percentage positive (+), and stain intensity (1+, 2+, 3+). Benign breast duct epithelium in the background serves as an internal barometer for stain intensity. Breast tumors are considered positive if 1% or more of tumor cells express ER. An important side note: if a case is negative for ER expression, and there is NO INTERNAL CONTROL (i.e. benign duct epithelium), which is positive, then repeat testing on the excision specimen is recommended. Block selection for testing should include benign background tissue, which can serve as an internal control.
In a breast case, if PR is positive and ER is negative, then the ER assay may not be working or the slides have been switched. PR expression should, practically, only occur in the setting of ER expression.
Marker Specificity
Sometimes ER is utilized as a marker to resolve the primary origin of an adenocarinoma. ER is relatively specific for breast origin, but far from perfect. Any “hormonally driven organ” (i.e. ovary, breast, uterus) may commonly show ER expression. Less commonly, practically any organ may show ER expression.
ER is often used as part of a panel to differentiate an endocervical adenocarcinoma (ER negative) from an endometrial adenocarcinoma (ER positive).
Common expression patterns in carcinoma (Dennis, JL, et al)
|
Tumor
|
Expression (%)
|
|
Breast
|
30-60%
|
|
Colon
|
<5%
|
|
Lung
|
<10%
|
|
Ovary
|
10-50%
|
|
Pancreas
|
0%
|
|
Stomach
|
<5%
|
|
Prostate
|
~10%
|
Microscopic Images
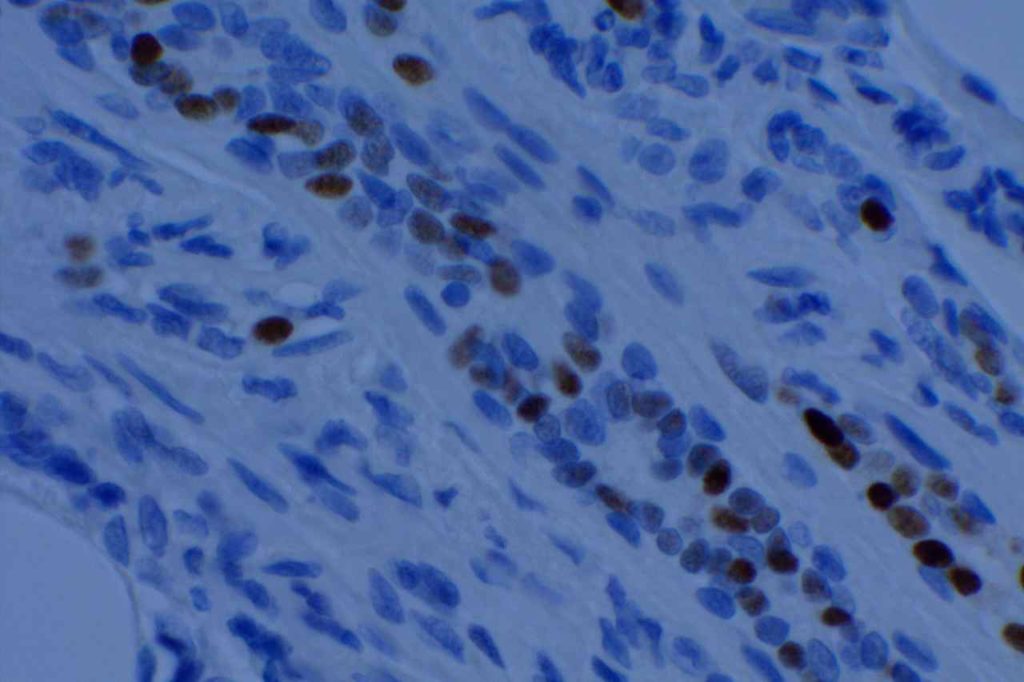
References
Yaziji, H., Taylor, C. R., Goldstein, N. S., Dabbs, D. J., Hammond, E. H., Hewlett, B., et al. (2008). Consensus recommendations on estrogen receptor testing in breast cancer by immunohistochemistry. Applied Immunohistochemistry & Molecular Morphology : AIMM / Official Publication of the Society for Applied Immunohistochemistry, 16(6), 513–520. doi:10.1097/PAI.0b013e31818a9d3a
Hammond, M. E. H., Hayes, D. F., Dowsett, M., Allred, D. C., Hagerty, K. L., Badve, S., et al. (2010). American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Archives of Pathology & Laboratory Medicine, 134(7), e48–72.
Dennis, J. L., Hvidsten, T. R., Wit, E. C., Komorowski, J., Bell, A. K., Downie, I., et al. (2005). Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research, 11(10), 3766–3772. doi:10.1158/1078-0432.CCR-04-2236