CD117 (c-kit) is a member of the type III tyrosine kinase receptor family, and is a transmembrane protein. Immunohistochemical expression of CD117 can be seen in many different normal tissue components (e.g. mast cells) and neoplastic tissues. Therefore, CD117 should be used in a careful targeted manner; usually as part of a larger panel. Limitations of specificity should always be kept in mind.
CD117 (c-kit) Expression Pattern:
- Gastrointestinal stromal tumors (~95%+)
- Mast cell neoplasms
- Seminiomas
- Small cell carcinomas / High grade neuroendocrine neoplasms
- PNETs
- Granulocytic sarcomas (small percentage, ~30%)
- Melanomas
- Myeolblasts (70% of CD34+ blasts in normal BMs);
- Plasma Cell Neoplasms
- Basal like breast carcinomas (~31%)
- Renal Tumors: Chromophobe RCC (83-100%, Oncocytoma (71-100%), negative in other subtypes of RCC
Hematopoietic
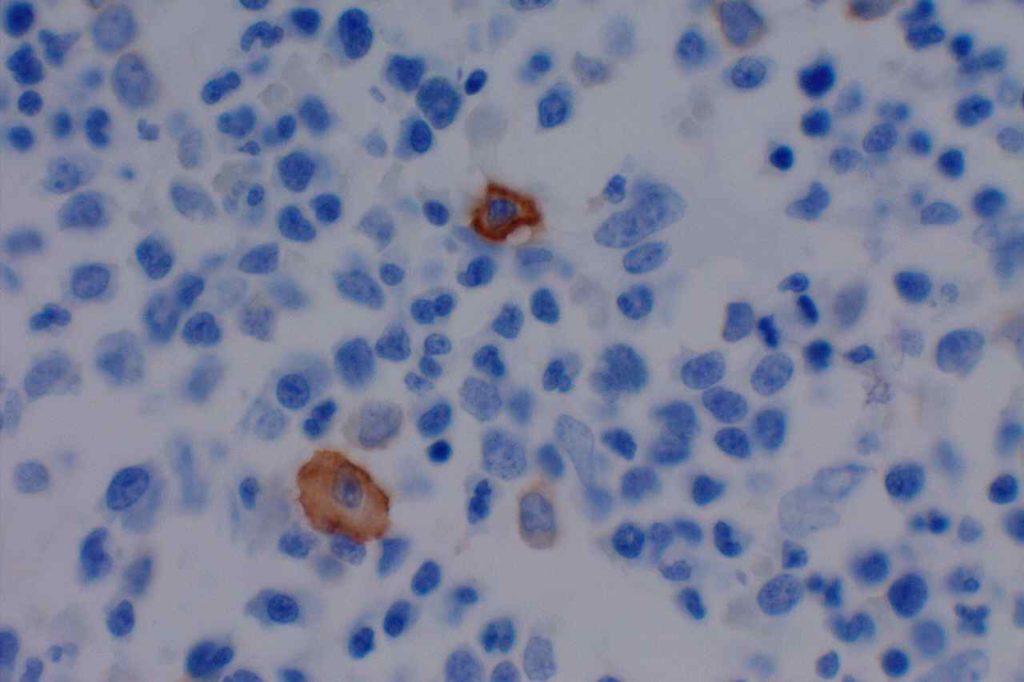
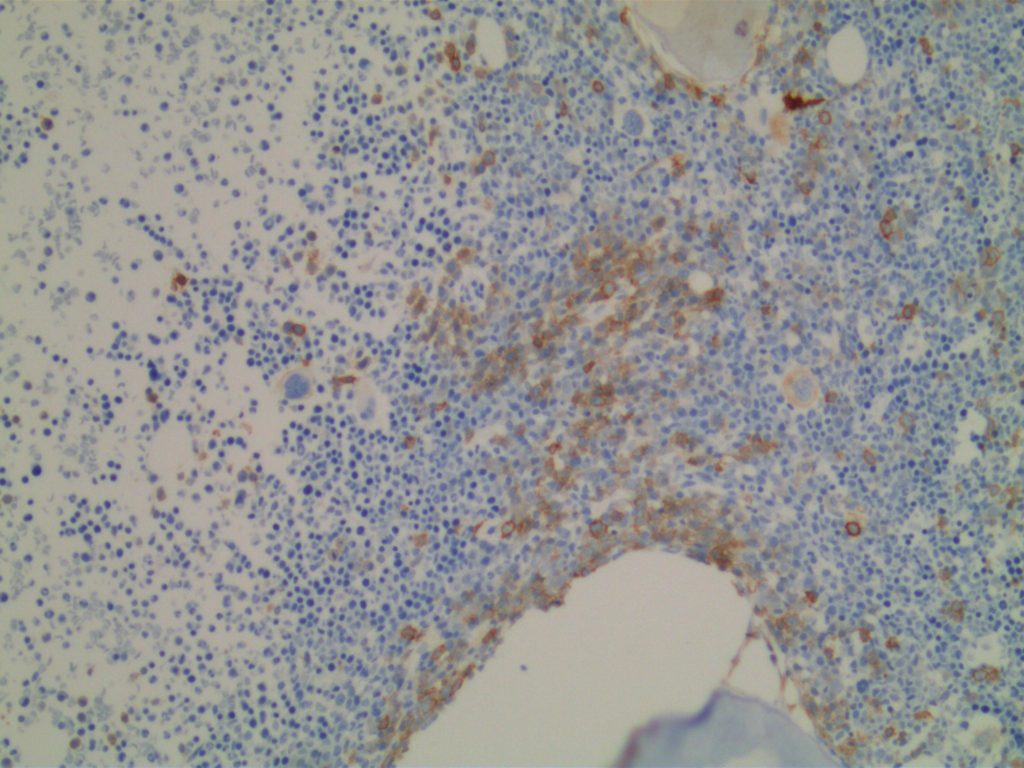
CD117 is most often used as a myeloblast marker in bone marrow biopsies, and to identify GISTs. It is important to understand what else may stain with CD117 (bright staining of mast cells in BM bxs., compared to dim staining of blasts), so one does not misinterpret the expression pattern. Erythroid precursor cell may also express CD117, which may cause confusion when evaluating for myeloblast percentage. Ohgami recommends using CD117, E-cadherin, and CD34 in combination in this situation. CD71 (erythroid precursor marker) may also be helpful.
Renal Tumors
CD117 has been found to be expressed in 71-100% of oncocytomas and 83-100% of chromophobe renal cell carcinomas with other subtypes of renal cell carcinoma being non-reactive. Therefore, CD117 may be a useful part of a larger panel (e.g. RCCMa, CD10, Vimentin, and cytokeratin) to diagnosis and subtype renal tumors (especially granular cell variant of RCC vs. chromophobe RCC or oncocytoma).
GIST
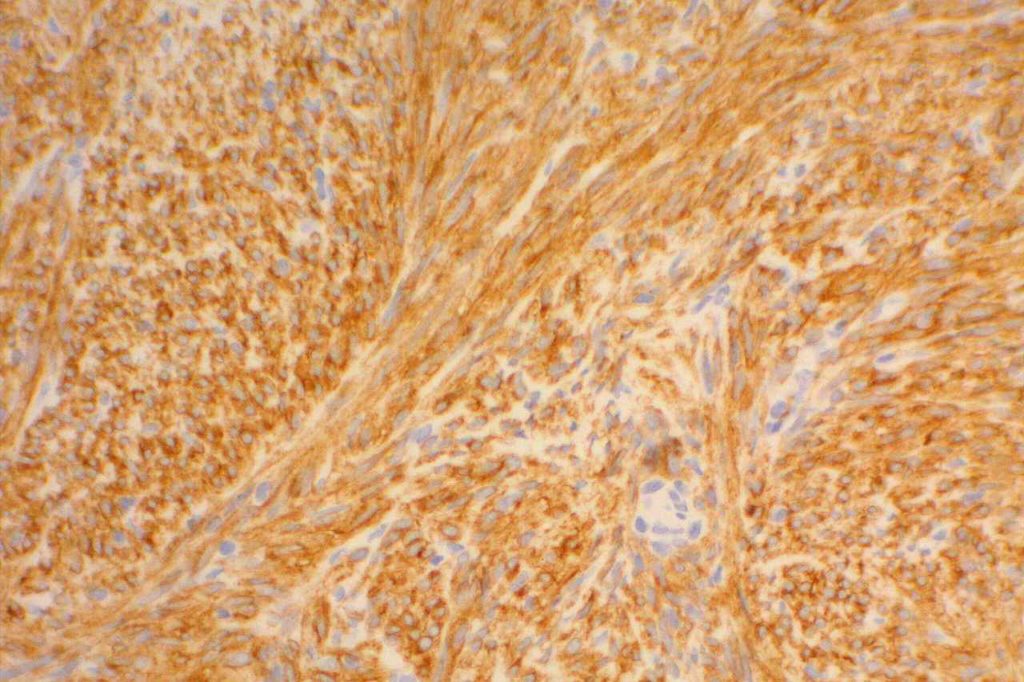
Approximately 95% of gastrointestinal stromal tumors (GIST) will react with CD117 by immunohistochemistry. It is important to emphasize that CD117 expression does not necessarily correlate with response to imatinib therapy (tyrosine kinase inhibitor). Lack of reactivity with CD117 should not exclude a patient from imatinib therapy. CD117 negative (kit-negative) GIST often contain PDGFRA mutations, which may be sensitive to imatinib. Sometimes weak expression of CD117 may be seen in non-GIST tumors, and this marker should be used as part of a larger panel incorporating the differential diagnosis (e.g. S-100, Desmin, CD34). DOG-1 antibody may be expressed in CD117 negative GIST cases, which may serve as an alternative marker.
Photomicrographs
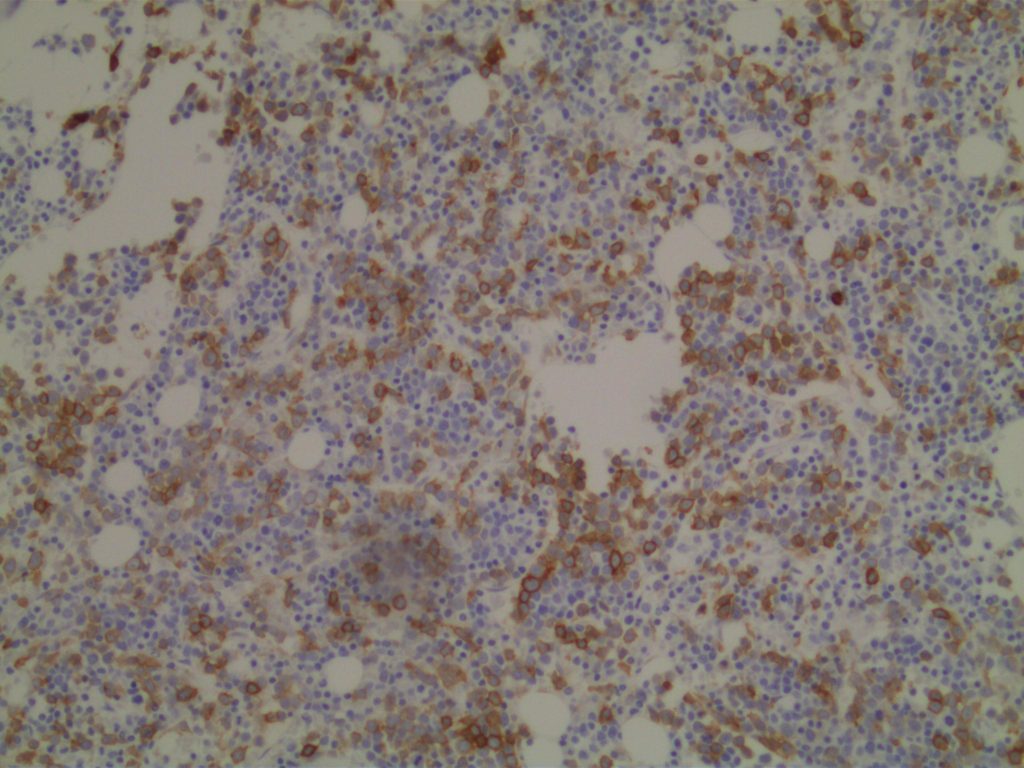
References:
Wick, MR. “Immunohistochemical approaches to the diagnosis of undifferentiated malignant tumor.”Annals of Diagnostic Pathology12(2008):72-84.
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 128.
Leidy, J., Khan, A., & Kandil, D. (2014). Basal-like breast cancer: update on clinicopathologic, immunohistochemical, and molecular features. Archives of Pathology & Laboratory Medicine, 138(1), 37–43. doi:10.5858/arpa.2012-0439-RA
Huang, Q., Wu, H., Nie, L., Shi, J., Lebenthal, A., Chen, J., et al. (2013). Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. The American Journal of Surgical Pathology, 37(4), 467–483. doi:10.1097/PAS.0b013e31826d2639
Dunphy, C. H., O’Malley, D. P., Perkins, S. L., & Chang, C.-C. (2007). Analysis of immunohistochemical markers in bone marrow sections to evaluate for myelodysplastic syndromes and acute myeloid leukemias. Applied Immunohistochemistry & Molecular Morphology : AIMM / Official Publication of the Society for Applied Immunohistochemistry, 15(2), 154–159. doi:10.1097/PAI.0b013e318030dec7
Patil, D. T., & Rubin, B. P. (2011). Gastrointestinal stromal tumor: advances in diagnosis and management. Archives of Pathology & Laboratory Medicine, 135(10), 1298–1310. doi:10.5858/arpa.2011-0022-RA
Bénet, C., Gomez, A., Aguilar, C., Delattre, C., Vergier, B., Beylot-Barry, M., et al. (2011). Histologic and immunohistologic characterization of skin localization of myeloid disorders: a study of 173 cases. American Journal of Clinical Pathology, 135(2), 278–290. doi:10.1309/AJCPFMNYCVPDEND0
Al-Ghawi, H., Asojo, O. A., Truong, L. D., Ro, J. Y., Ayala, A. G., & Zhai, Q. J. (2010). Application of Immunohistochemistry to the Diagnosis of Kidney Tumors. Pathology Case Reviews, 15(1), 25–34. doi:10.1097/PCR.0b013e3181d51c70
Liegl-Atzwanger, B., Fletcher, J. A., & Fletcher, C. D. M. (2010). Gastrointestinal stromal tumors. Virchows Archiv : an International Journal of Pathology. doi:10.1007/s00428-010-0891-y
Siegert, S. I., Diebold, J., Ludolph-Hauser, D., & Löhrs, U. (2004). Are gastrointestinal mucosal mast cells increased in patients with systemic mastocytosis? American Journal of Clinical Pathology, 122(4), 560–565. doi:10.1309/2880-LF7Q-6XK3-HA3Q
Antonescu, C. R. (2008). Targeted therapy of cancer: new roles for pathologists in identifying GISTs and other sarcomas. Modern Pathology : an Official Journal of the United States and Canadian Academy of Pathology, Inc, 21 Suppl 2, S31–6. doi:10.1038/modpathol.2008.9
Medeiros, F., Corless, C. L., Duensing, A., Hornick, J. L., Oliveira, A. M., Heinrich, M. C., et al. (2004). KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. The American Journal of Surgical Pathology, 28(7), 889–894.
Wang, H.-Y., & Mills, S. E. (2005). KIT and RCC are useful in distinguishing chromophobe renal cell carcinoma from the granular variant of clear cell renal cell carcinoma. The American Journal of Surgical Pathology, 29(5), 640–646.
Ohgami, R. S., Chisholm, K. M., Ma, L., & Arber, D. A. (2014). E-Cadherin Is a Specific Marker for Erythroid Differentiation and Has Utility, in Combination With CD117 and CD34, for Enumerating Myeloblasts in Hematopoietic Neoplasms. American Journal of Clinical Pathology, 141(5), 656–664. doi:10.1309/AJCP8M4QQTAZPGRP