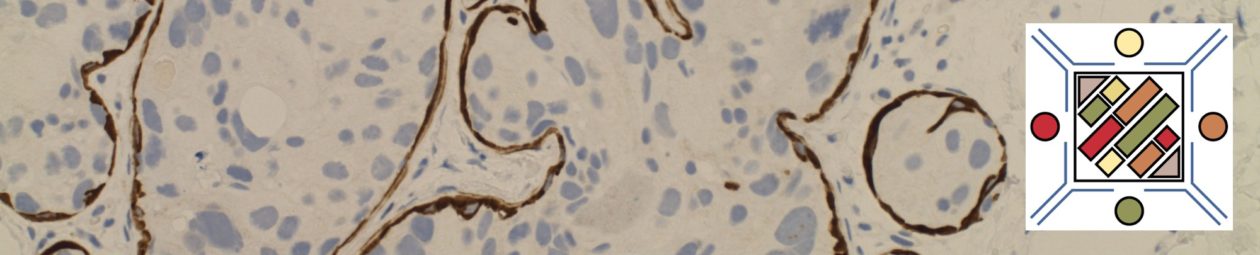
- PDGFA
- Eosinophilia with increased tryptase and marrow mast cells
- Cryptic deletion in chromosome 4q12
- FIP1L1-PDGFA, many other partners
- Responsive to tyrosine kinase inhibitors (e.g. imatimib/Gleevec®)
- PDGFB
- Eosinophilia combined with monocytosis that mimics CMML
- t(5;12)(q31;33;p12) ETV6-PDGFB, many other partners
- Responsive to TKIs
- FGFR1
- PCM1-JAK2 (provisional entity)
- Eosinophilia along with acute lymphoblastic leukemia (B-cell) or lymphoma (T-cell)
- Bone marrow with lymphoid aggregates and increased/left shifted erythroid precursors (mimics PMF)
- May respond to JAK2 inhibitors (e.g ruxolitinib/Jakafi®)
References
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127: 2391–2405. doi:10.1182/blood-2016-03-643544
Swerdlow SH, Campo E, Harris, NL, Jaffe ES, Pileri SA, Stein H, Thiele J (Eds): WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th edition). IARC: Lyon 2017