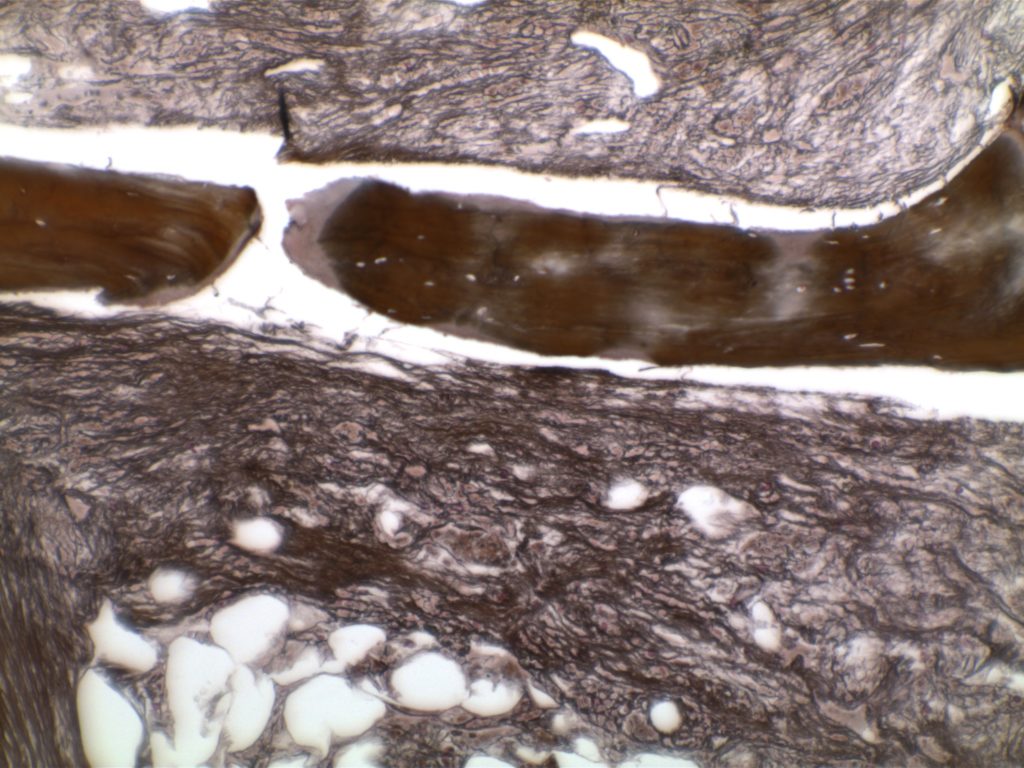
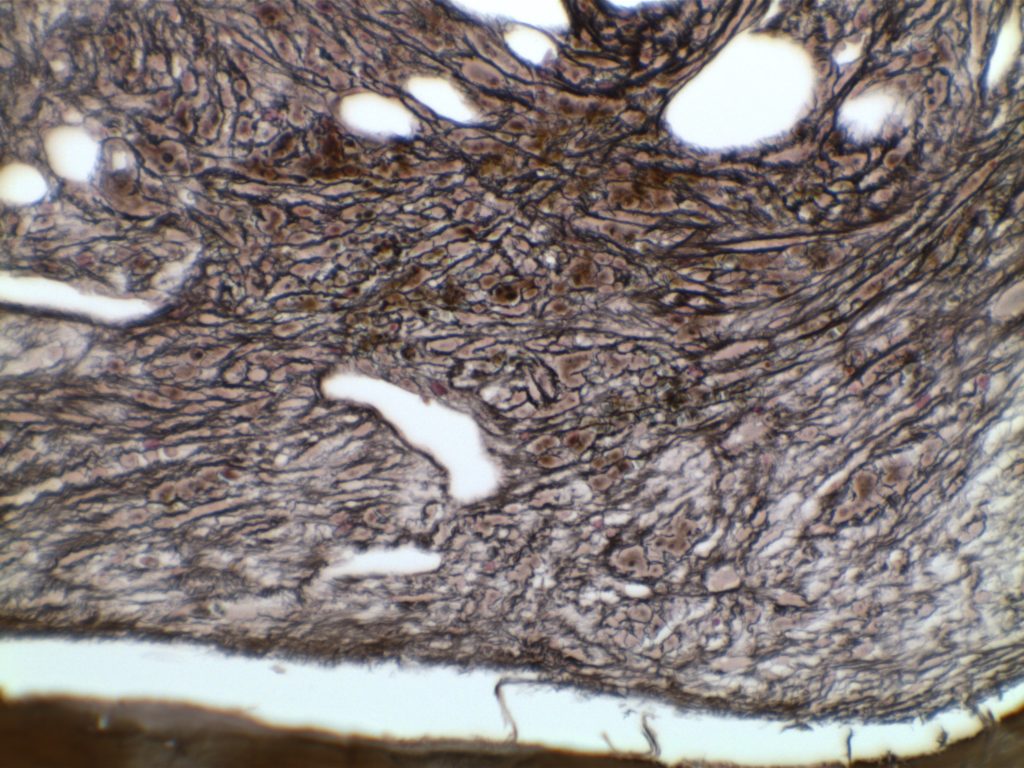
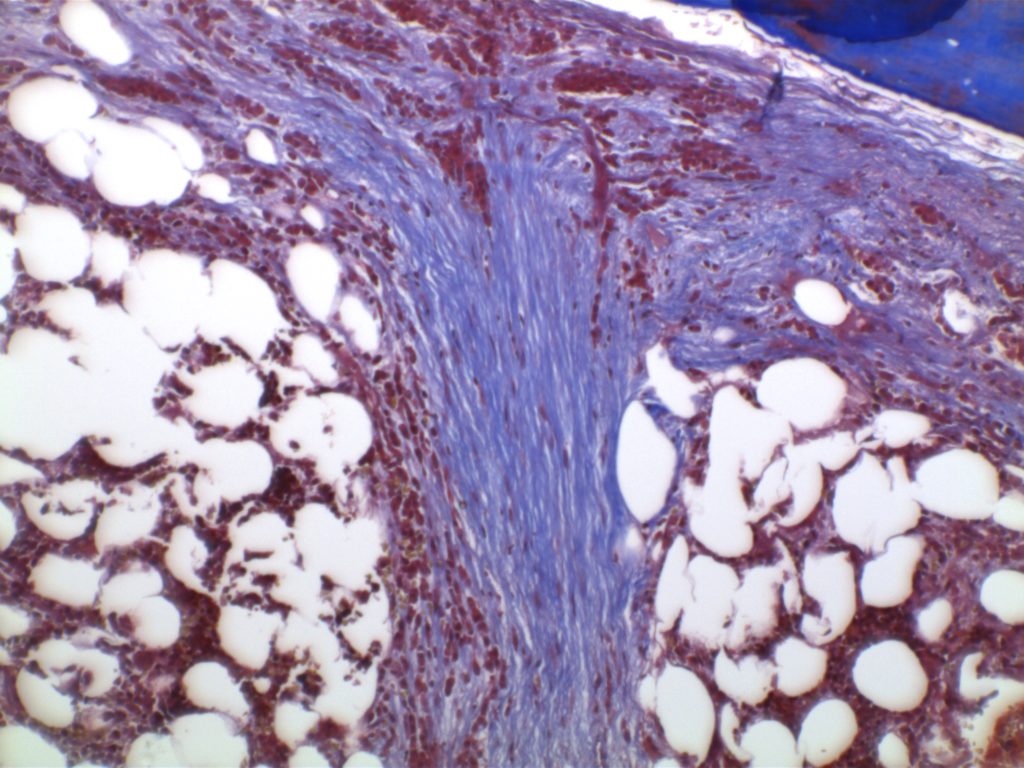
Myelofibrosis is characterized by (typically) increased reticulin fibrosis or (less commonly) collagen fibrosis (trichrome stain). Hematologic malignancies are often leading culprits, but consideration of other etiologies should be considered.
The following categories and entities should be considered with the finding of myelofibrosis.
Infectious diseases
- Tuberculosis
Autoimmune disorders
- Systemic lupus erythematosus (SLE)
- Sjogren’s syndrome (SS)
- Systemic sclerosis
- Primary autoimmune myelofibrosis
- Connective tissue disease
Drug associated conditions
- Thrombopoietin receptor agonist toxicity
Endocrine disorders
- Hyperparathyroidism (primary or secondary)
- Vitamin D deficiency (nutritional or rickets)
- Osteomalacia
Hematologic malignancies
- Myelodysplastic syndrome (MDS)
- Myeloproliferative neoplasms (MPN)
- Chronic myelogenous leukemia (CML)
- Hodgkin lymphoma
- Non-Hodgkin lymphoma
- Acute myeloid leukemia (AML) – particularly acute megakaryoblastic leukemia
- Acute lymphoblastic leukemia (ALL)
- Adult T-cell leukemia/lymphoma (ATLL)
- Multiple myeloma (MM)
- Systemic mastocytosis
Other hematologic malignancies
- Paroxysmal nocturnal hemoglobinuria (PNH)
- Gray platelets syndrome
Other
- Primary hypertrophic osteoarthropathy
- Paget disease
- Metastatic solid tumor malignancies
Myelofibrosis Grading
|
Grade
|
Comment
|
|
Scattered linear fibers without intersections. Normal bone marrow.
|
|
|
MF1
|
Loose network of reticulin fibers with intersections (particularly perivascular)
|
|
Diffuse increase of reticulin fibers with increased density and numerous intersections. Focal bundles of thick fibers.
|
|
|
Diffuse increase of reticulin fibers with increased density and numerous intersections. Increased thick bundles of fibers consistent with collagen fibrosis. Osteosclerosis usually present.
|
In cases of MF2 or MF3, it is recommended to perform trichrome stain to evaluate for collagen fibrosis.
References
Marcellino B, Jamal El SM, Mascarenhas JO. Distinguishing autoimmune myelofibrosis from primary myelofibrosis. Clin Adv Hematol Oncol. 2018;16: 619–626.