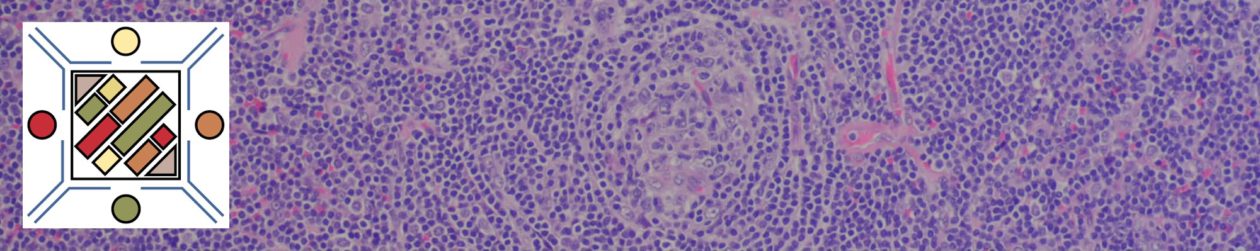
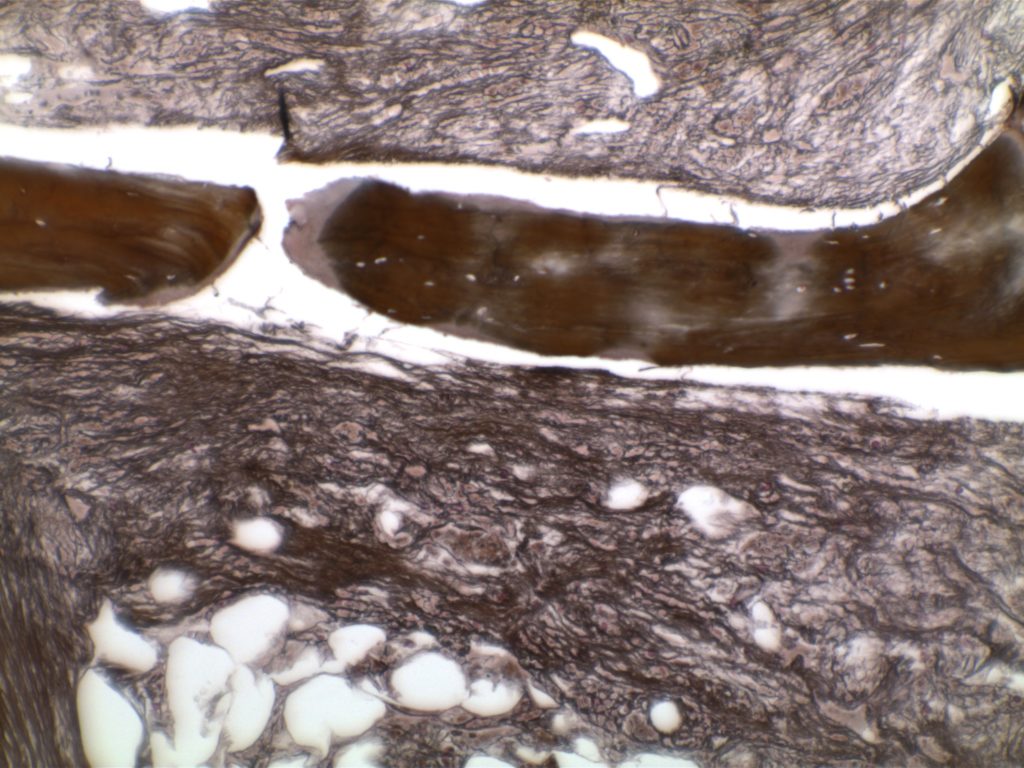
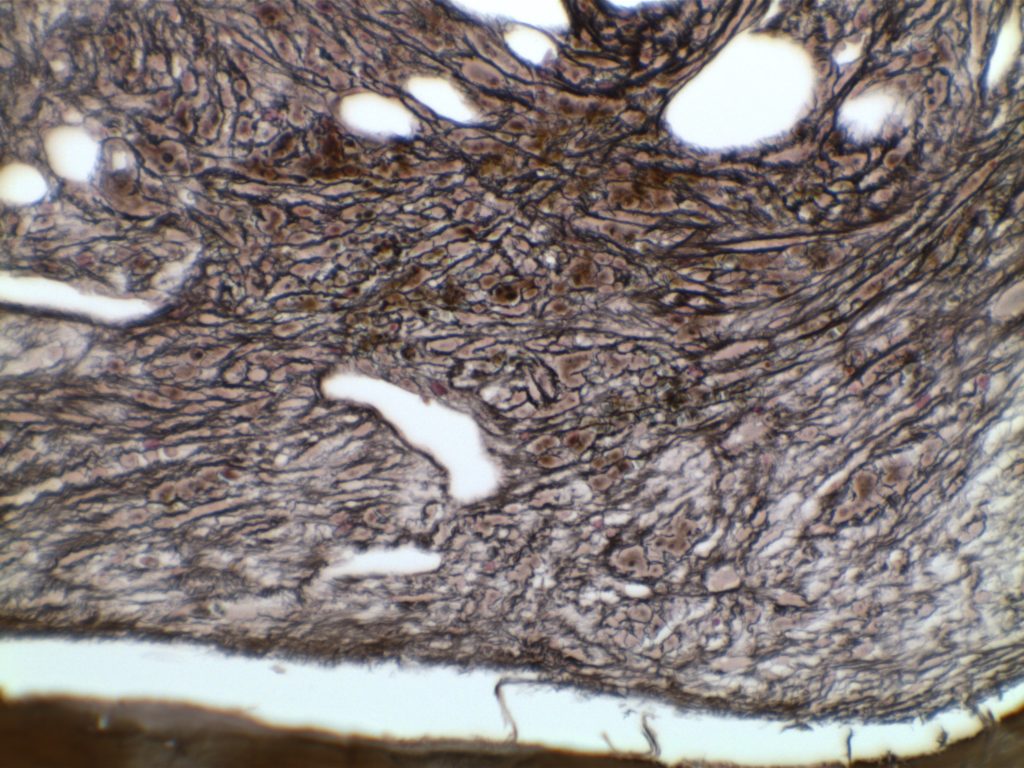
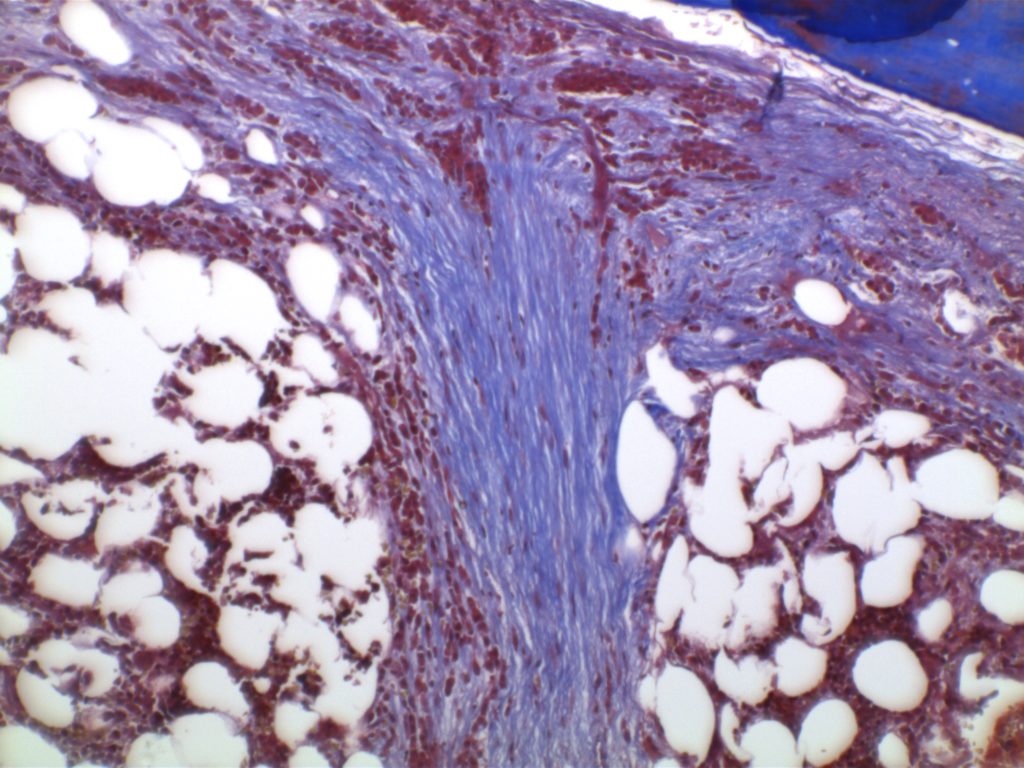
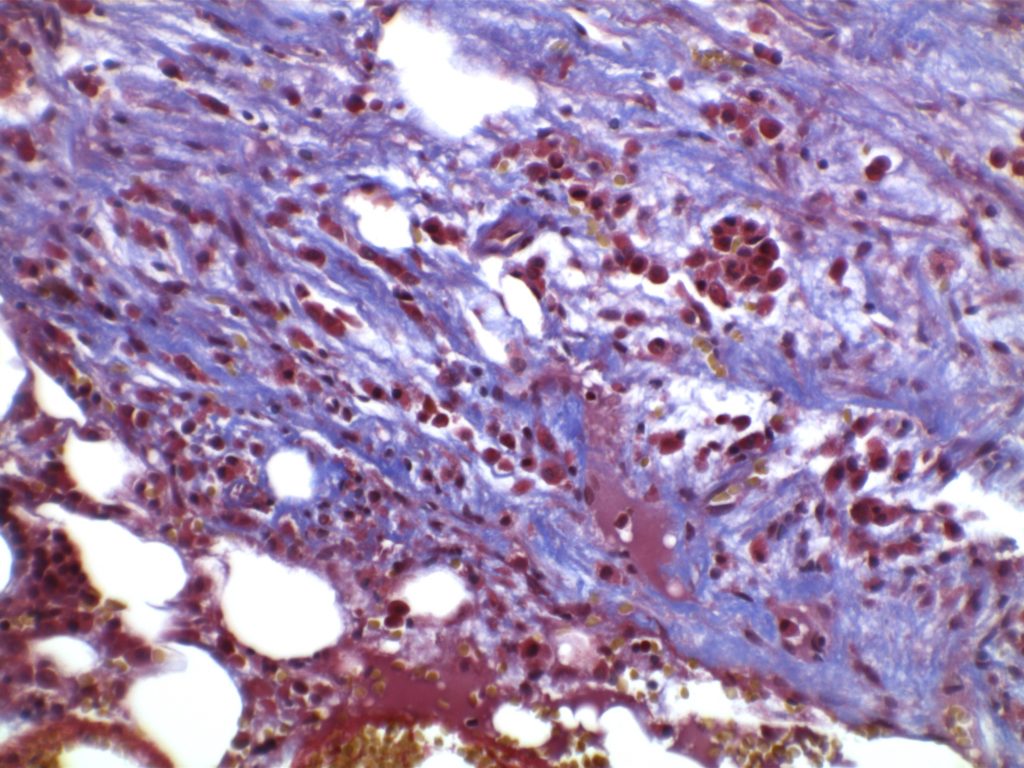
The anaplastic variant of diffuse large B-cell lymphoma (DLBCL) is a morphologic variant under the DLBCL, NOS category in the WHO Classification. Typical common features include:
- Marked nuclear pleomorphism, often confused with poorly differentiated carcinoma.
- Large and significantly enlarged neoplastic cells.
- May resemble Hodgkin lymphoma or anaplastic large cell lymphoma (ALCL).