In Situ Follicular Neoplasia (ISFN), which has been previously referred to as Follicular Lymphoma Is Situ (FLIS) is found in approximately 2% of otherwise reactive lymph nodes. ISFN is defined as partial to total colonization of germinal centers by B-cells containing the t(14;18) BCL-2/IgH translocation characteristic of follicular lymphoma.
ISFN found as an incidental finding have a risk of developing follicular lymphoma (FL) of <= 5%. The amount of involvement of ISFN within a lymph node does not appear to be directly related to the risk of future FL.
If patients have other evidence of lymphadenopathy, then additional biopsies may be helpful to exclude a B-cell lymphoma at another site.
Morphology
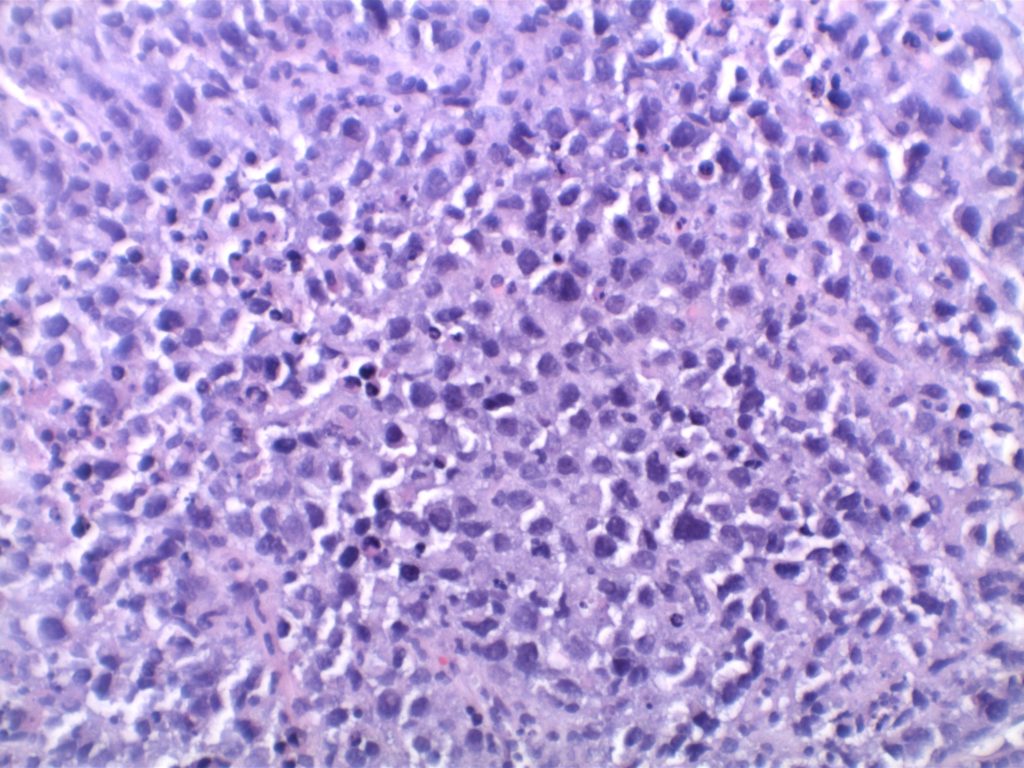
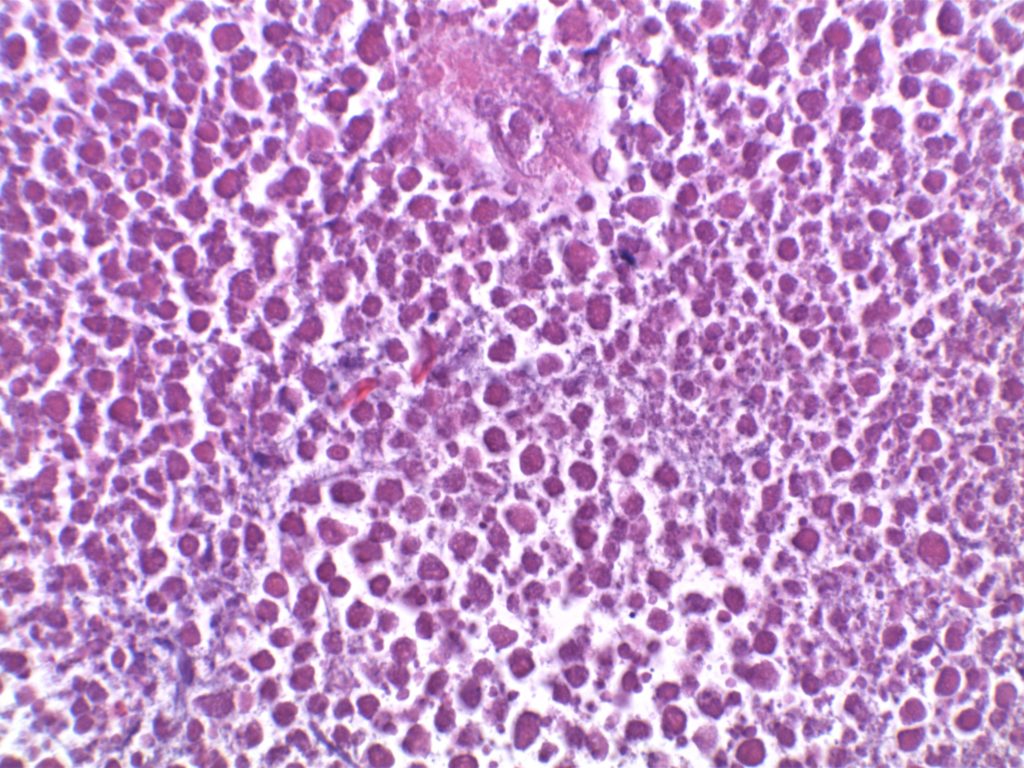
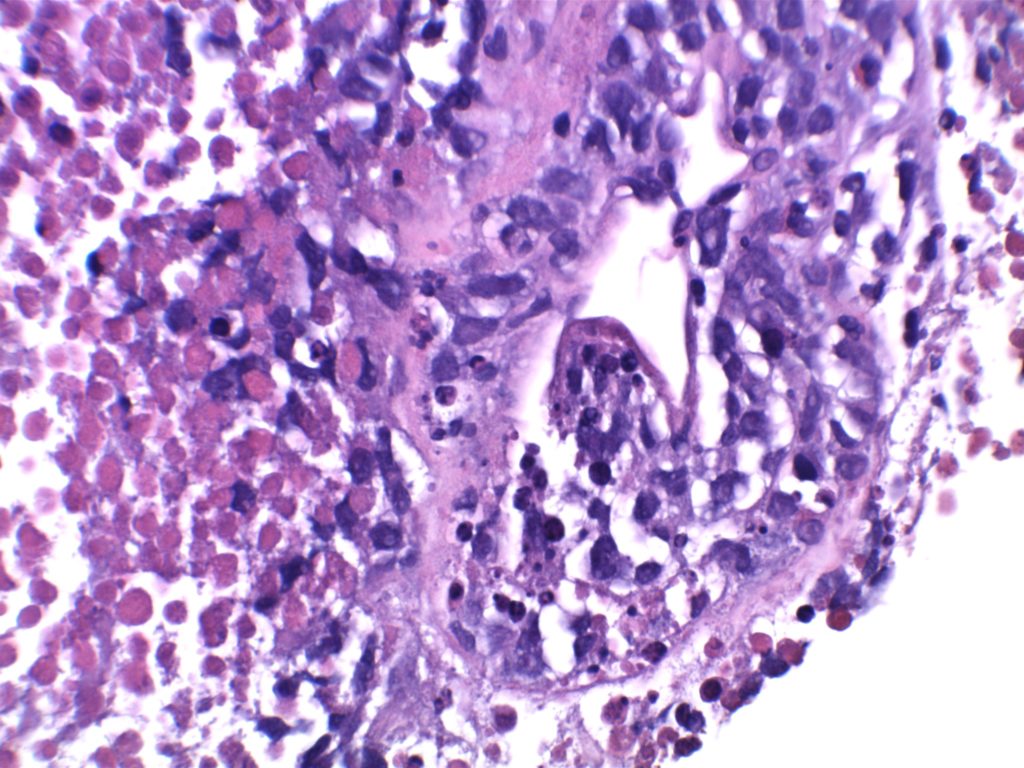
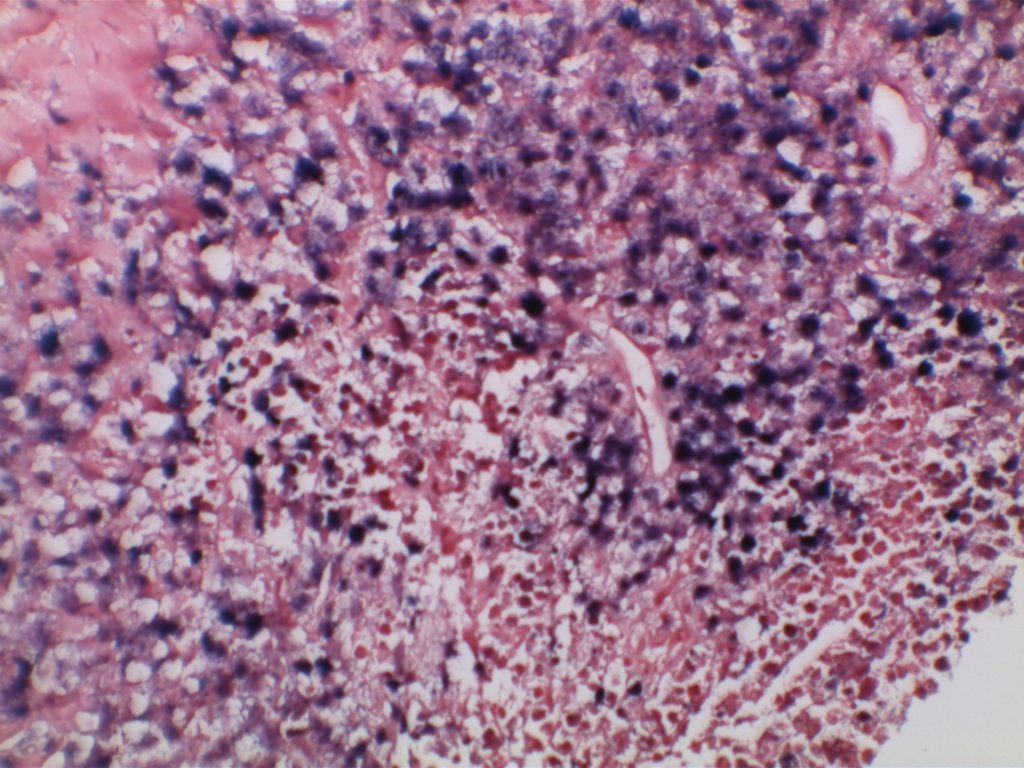
H&E staining demonstrates normal appearing lymph node architecture with well-formed and well-demarcated germinal centers. Follicles with ISFN at first glance do not look significantly different from the adjacent reactive follicles. Some areas of ISFN may more prominent and closely associated centrocytes (subtle).
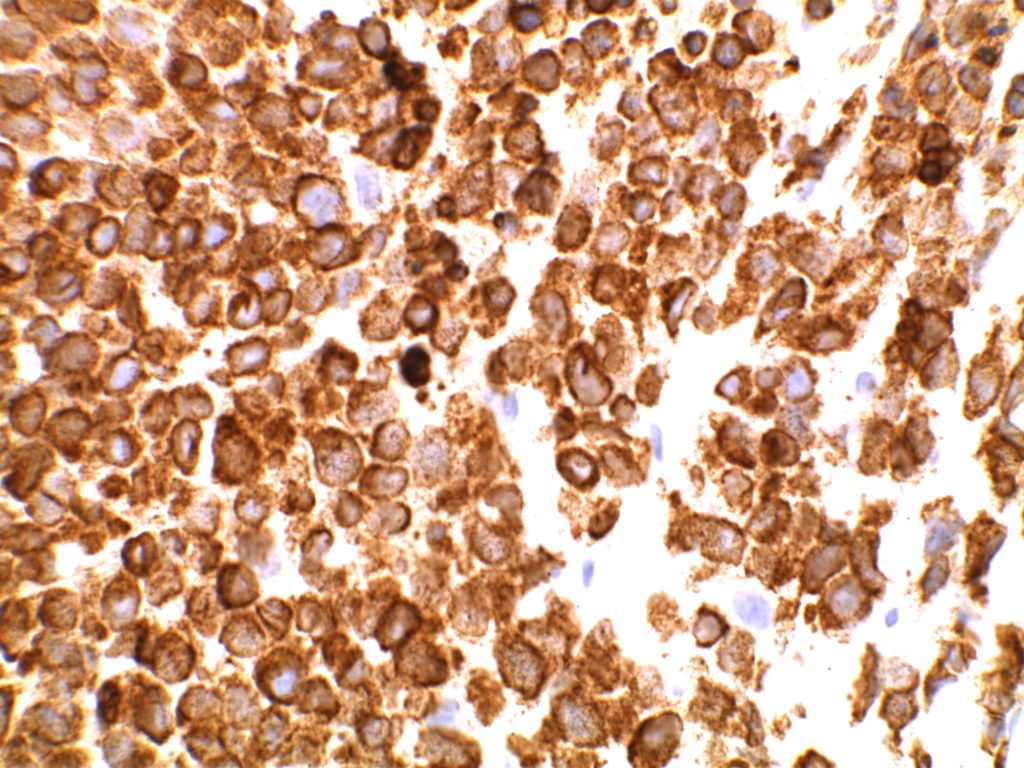
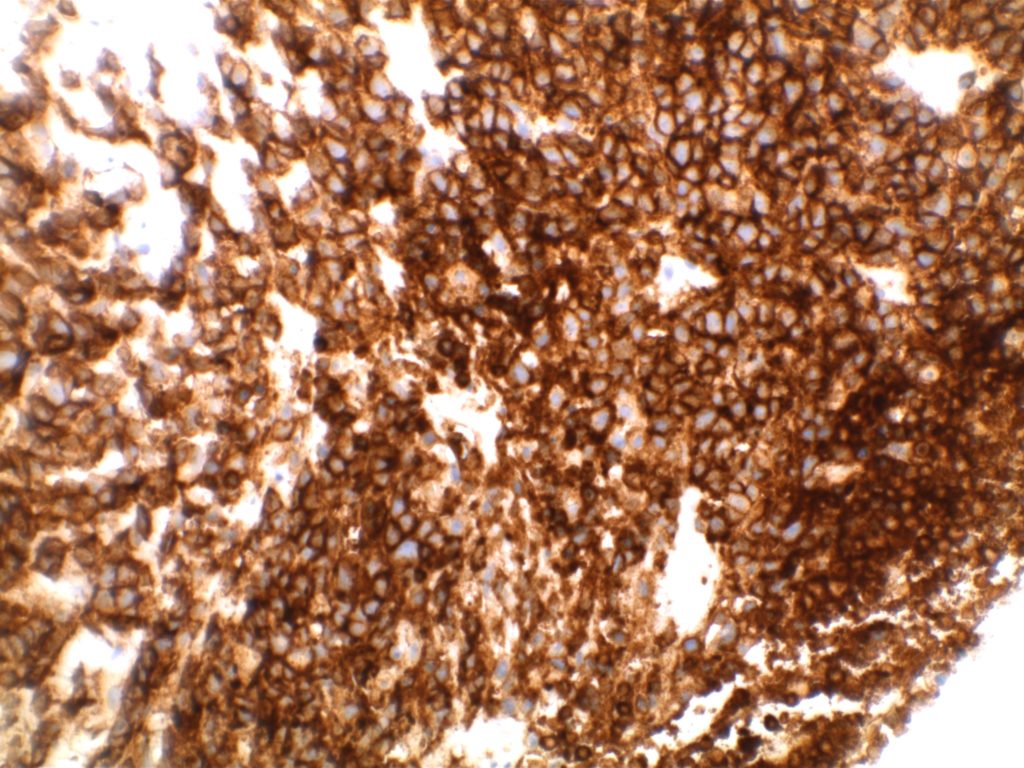
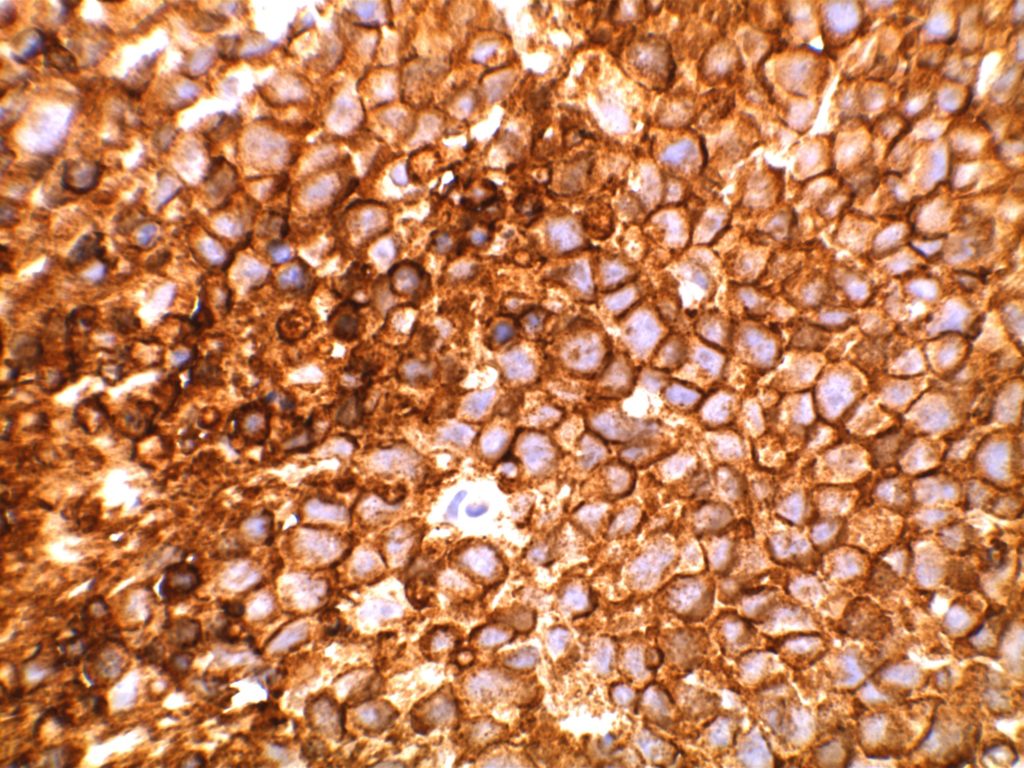
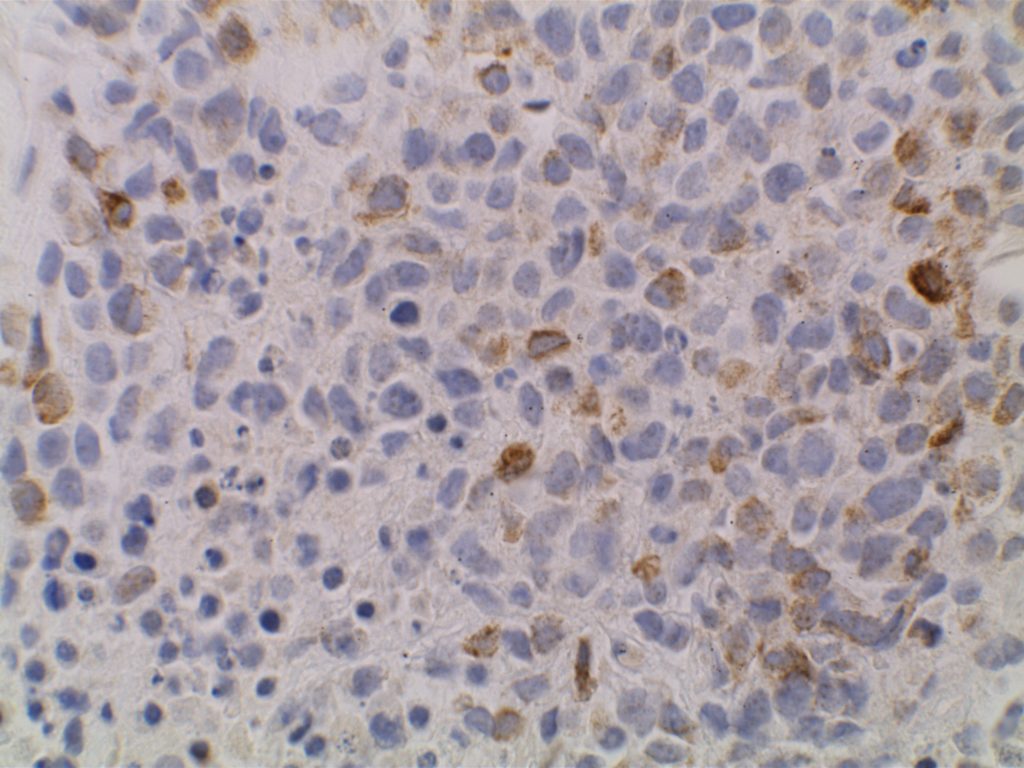
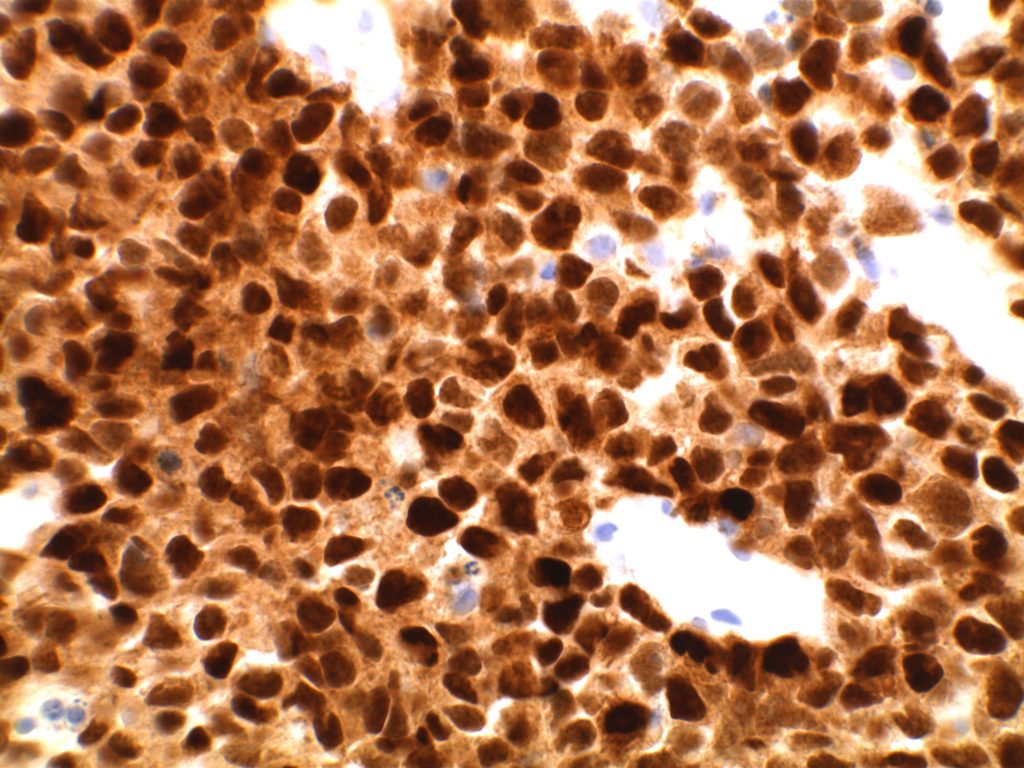
Immunohistochemistry
- CD10 – Strong expression
- Bcl-6 – Positive
- Bcl-2 – Positive (often stronger expression than surrounding lymphocytes)
- Ki-67 – Usually significantly lower expression than that of adjacent reactive germinal centers
- CD20 – Positive
Photomicrographs
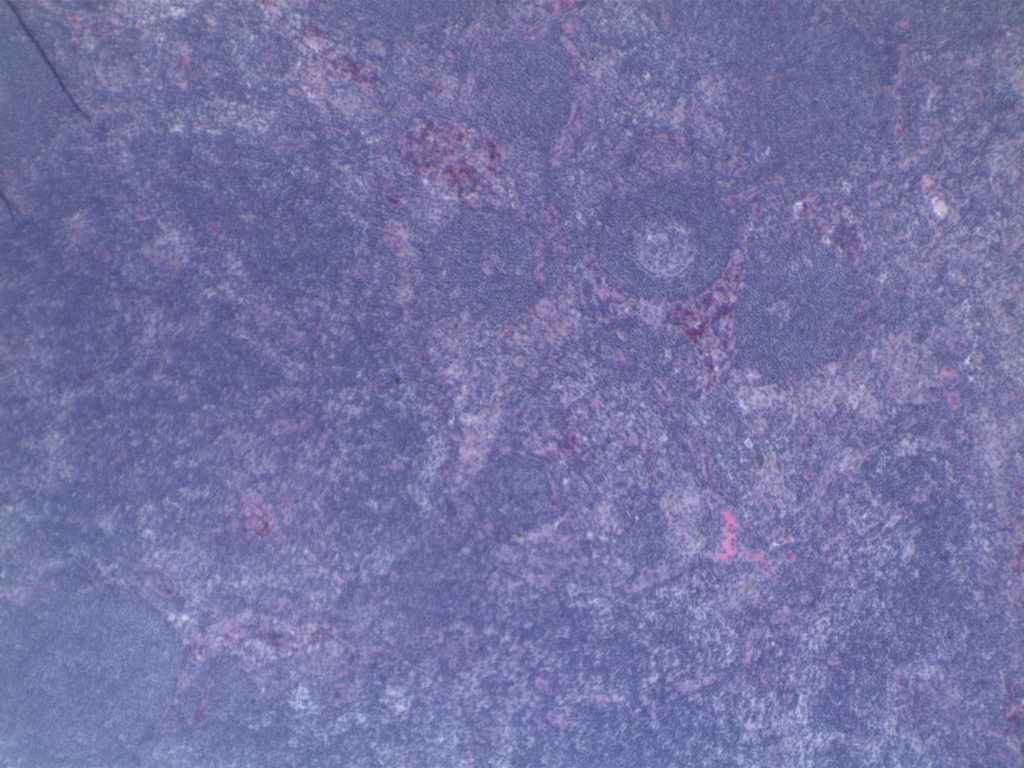
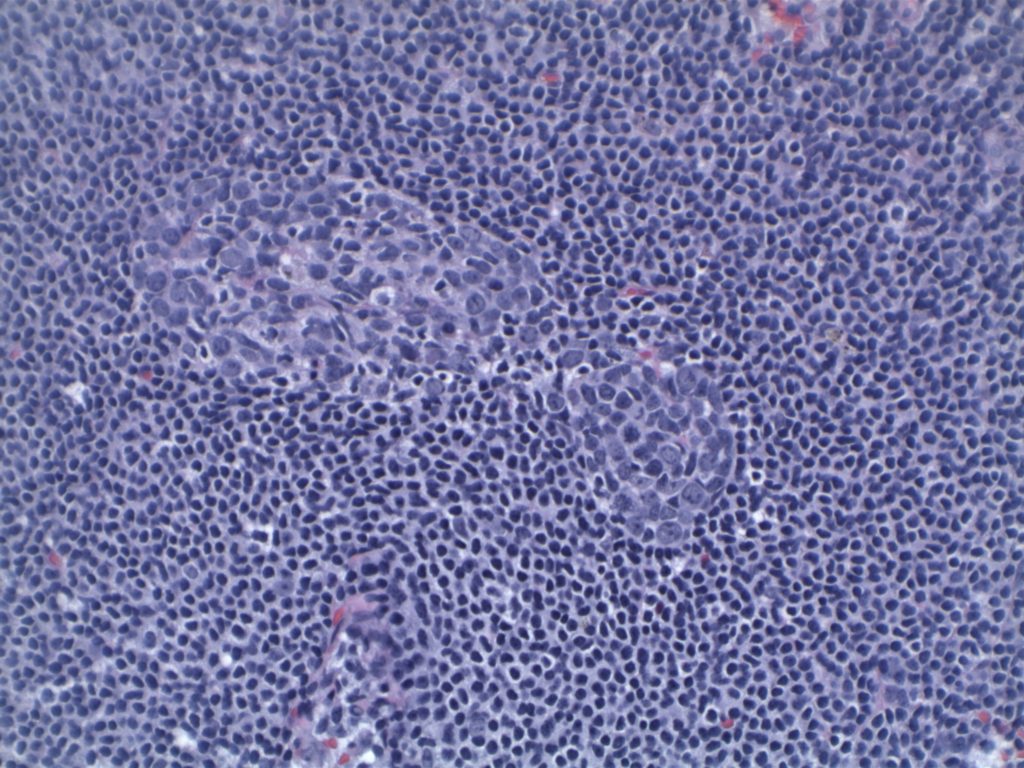
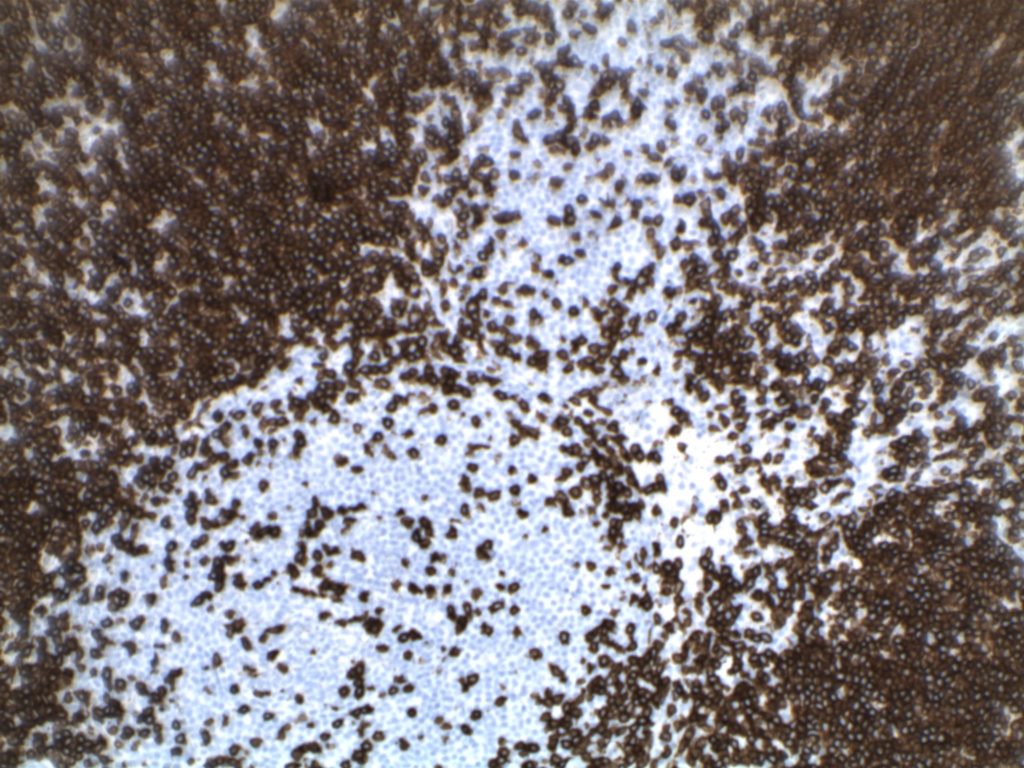
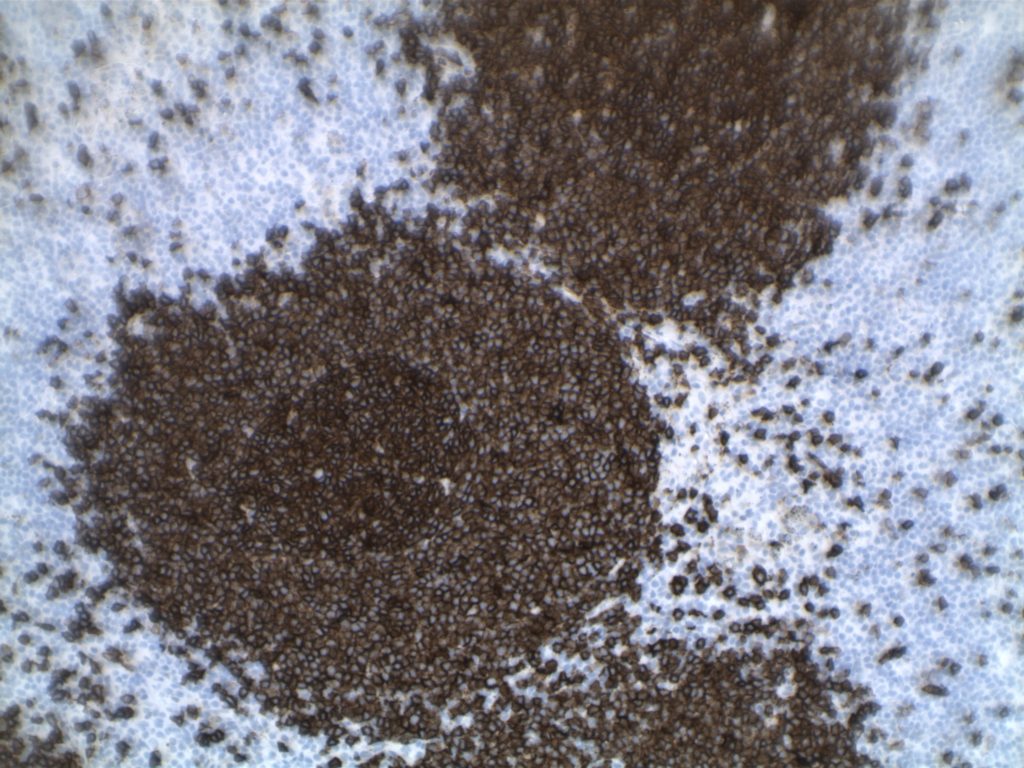
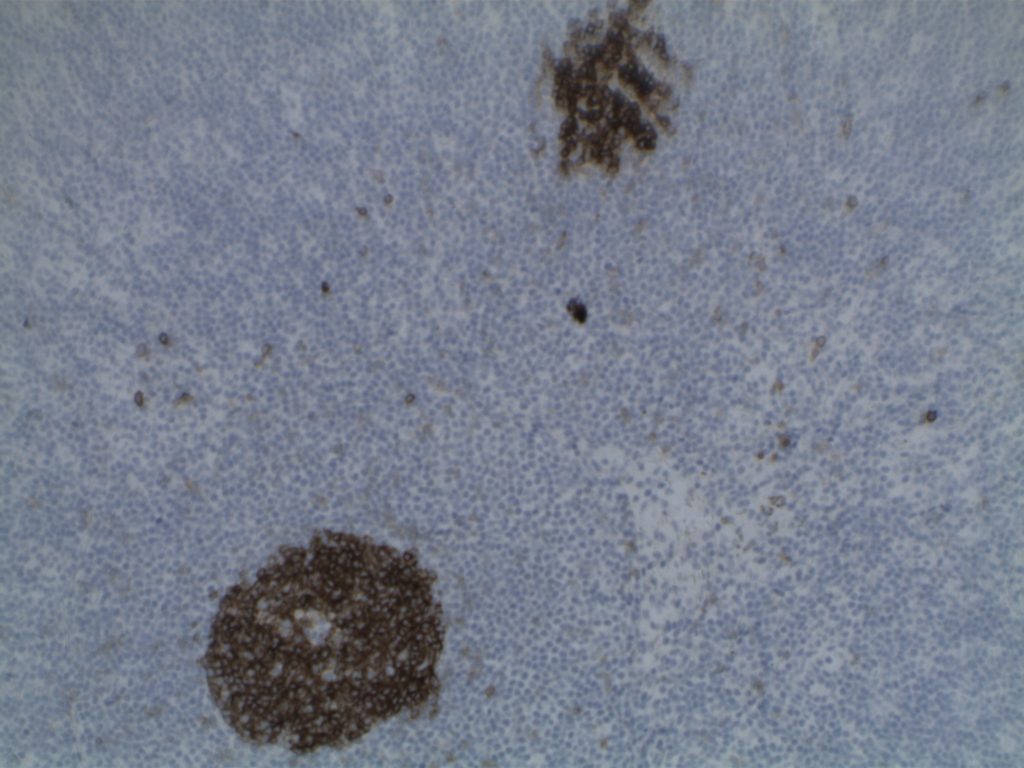
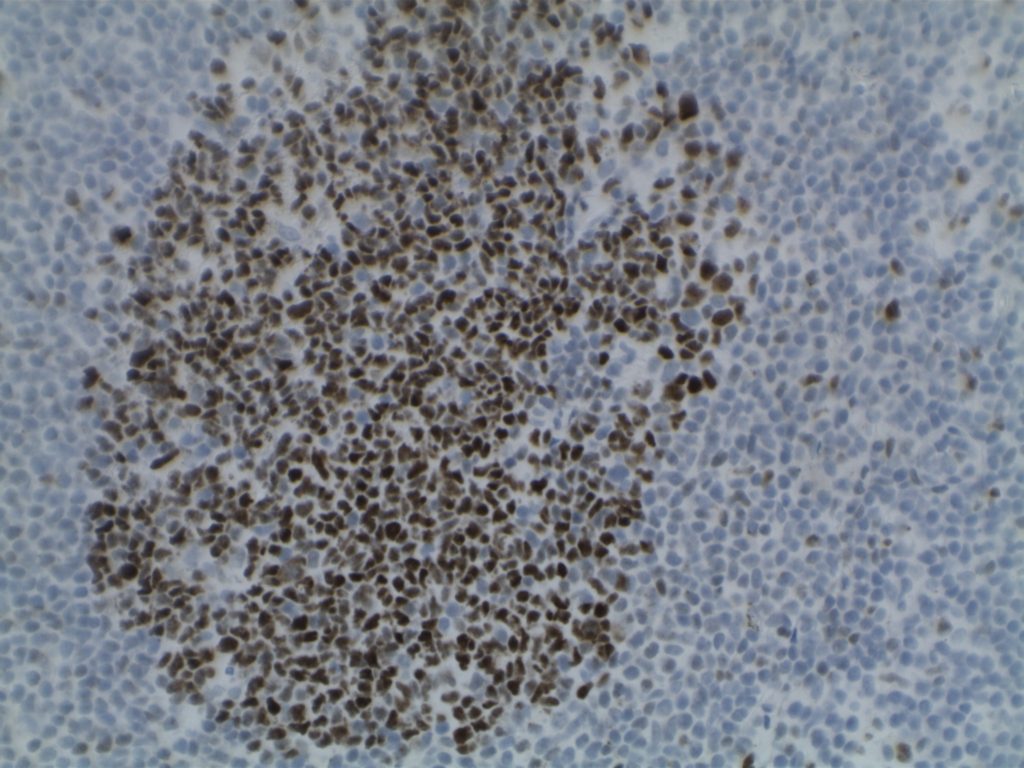
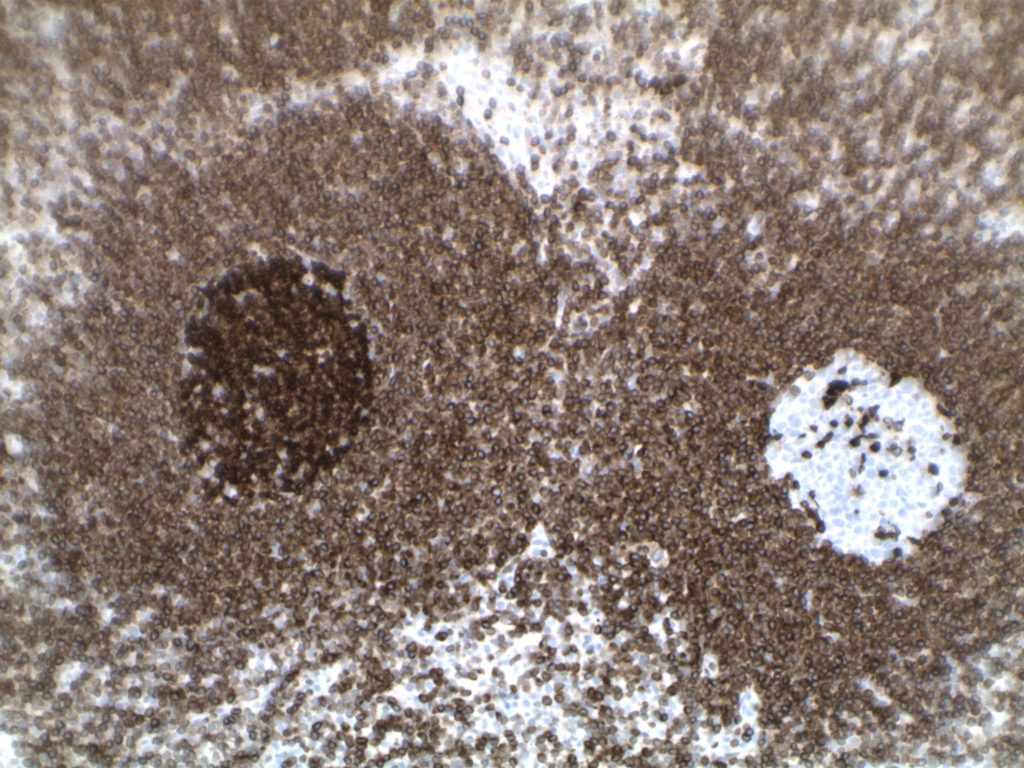
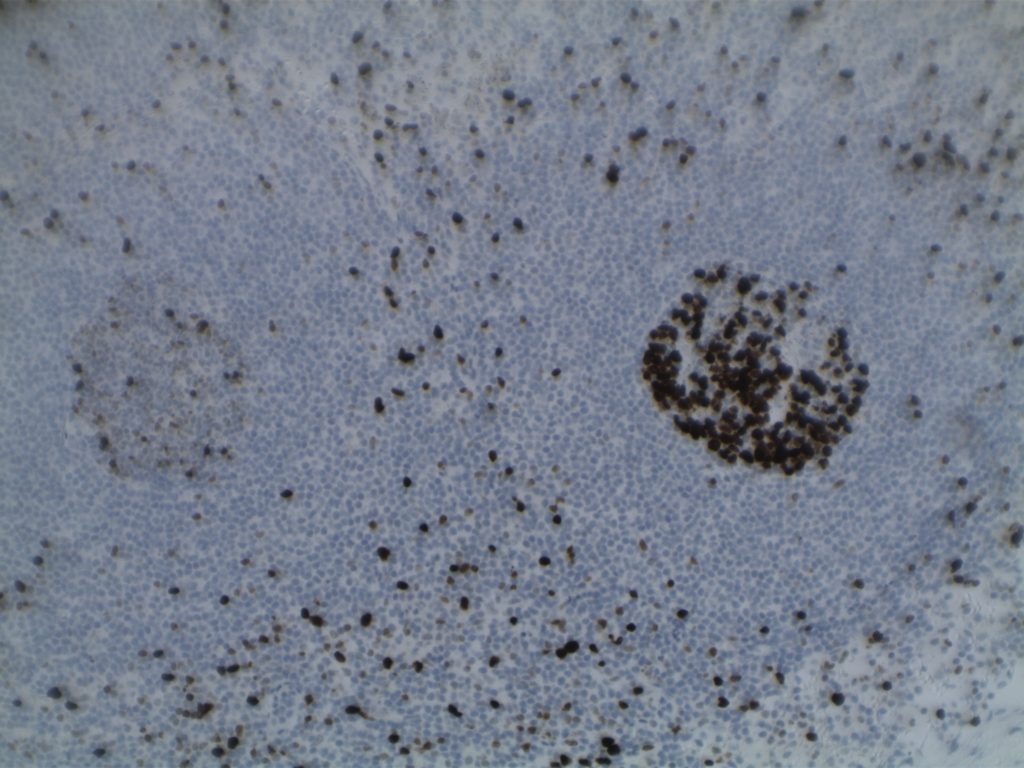
Differentiating from partial involvement by follicular lymphoma
Sometimes it can be difficult to differentiate partial involvement of a lymph node by FL from ISFN. The following features are helpful to differentiate partial involvement by FL from ISFN:
- ISFN – Intact nodal architecture
- Partial involvement by FL – Altered architecture on H&E sections
- ISFN – Normal sized follicles
- Partial involvement by FL – Often enlarged follicles
- ISFN – Sharp border between the follicle center and the surrounding mantle zone
- Partial involvement by FL – Interface between the follicle center and mantle zone is often fuzzy or blurred.
- ISFN – Bcl-2 is strongly positive (usually brighter than the surrounding T-cells and mantle zones); CD10 strongly positive
- Partial involvement by FL – variable Bcl-2 and CD10 expression
- ISFN – Atypical cells are confined to the follicle center
- Partial involvement by FL – atypical cells (CD10/bcl2+) can be found outside of the follicle center areas
References
Swerdlow SH, Campo E, Harris, NL, Jaffe ES, Pileri SA, Stein H, Thiele J (Eds); WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th edition). IARC: Lyon 2017