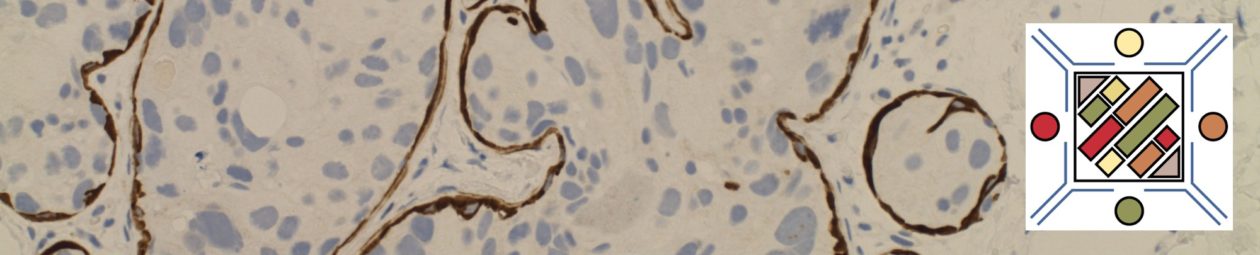
Lymphoplasmacytic Lymphoma (LPL)
- Neoplastic proliferation of B-cells ranging in spectrum from lymphocytes to plasma cells (typically involves the bone marrow, but can also involve the spleen and lymph nodes).
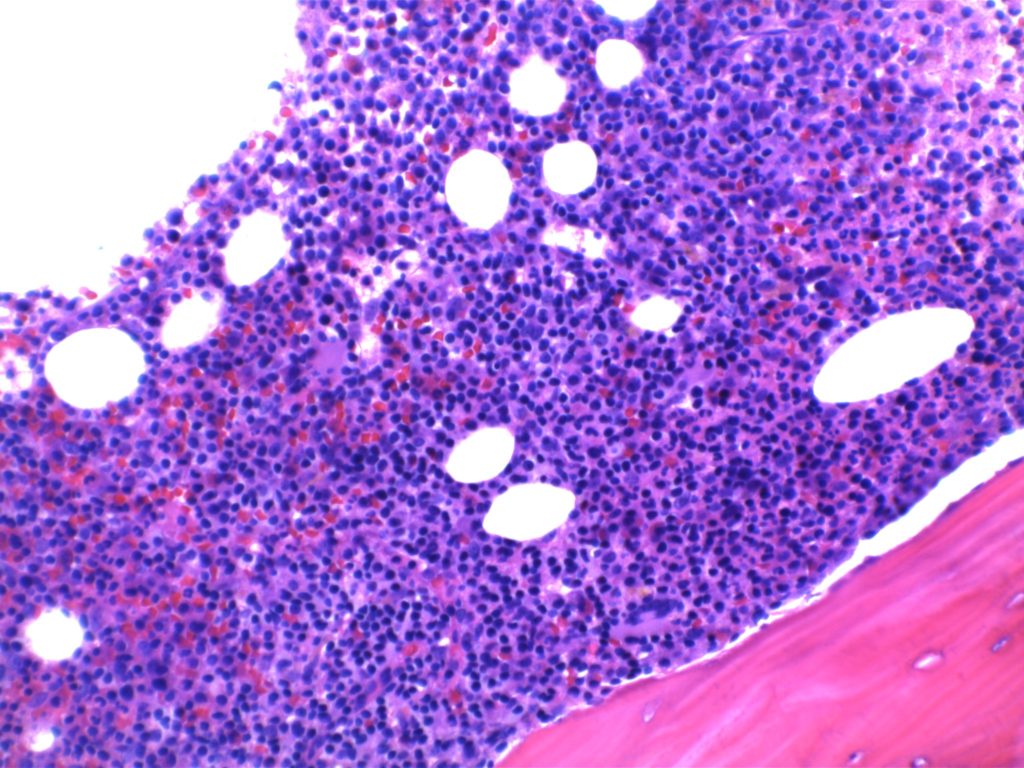
- Bone marrow involvement
- Nodular, diffuse, and/or interstitial
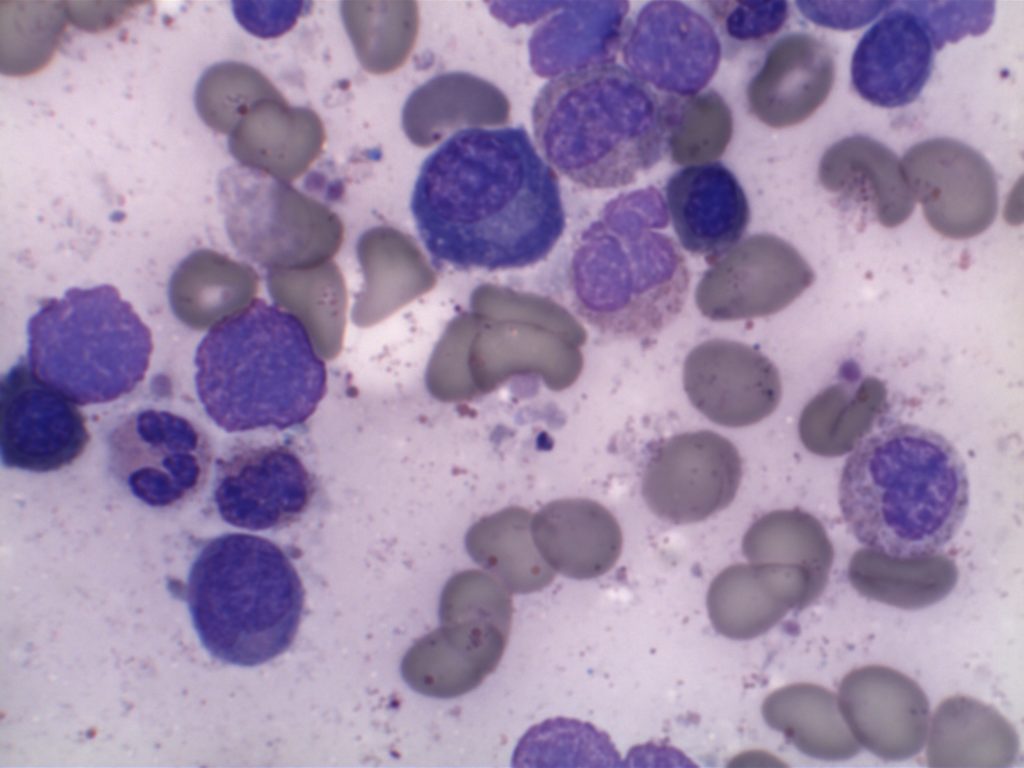
- Small lymphocytes admixed with plasma cells and plasmacytoid lymphocytes
- IgM paraprotein
- Sometimes result in a hyperviscosity syndrome(30%)
- IgM and IgG paraprotein (minority)
- Some cases may be IgG or IgA.
- Cryoglobulinemia (20% of WM)
- Coagulopathy (IgM binds to clotting factors)
- MYD88 (L265P) point mutation (>90%)
- Results in up-regulation of NF-κB (promotes tumor cell survival).
- Not specific for LPL.
- LPL is a diagnosis of exclusion after other B cell lymphoid neoplasms with plasmacytic differentiation have been excluded.
- IgM MGUS is now thought to be more closely related to LPL than plasma cell myeloma.
Most patients present with non-specific B-symptoms. However, ~10% have hemolysis secondary to cold agglutinins (IgM binds to RBCs at temperature <37C).
Waldenstrom macroglobulinemia
Waldenstrom macroglobulinemia (defined as bone marrow involvement by LPL and IgM monoclonal gammopathy) may manifest as a hyperviscosity syndrome due to the circulating IgM in the blood (IgM tends to stay in the vascular system, compared to IgG which can penetrate into soft tissue). Because IgM molecules tend to be well-segregated to the vascular compartment, plasmapheresis can be very effective to treat hyerpviscosity symptoms. Clinical signs/symptoms include:
- Cryoglobulinemia – precipitation of macroglobulins as temperatures <37C
- Bleeding – macroglobulins interfere with clotting factors
- Neurologic disturbances – secondary to increased blood viscosity
- Visual impairment – secondary to increased viscosity and hemorrhage
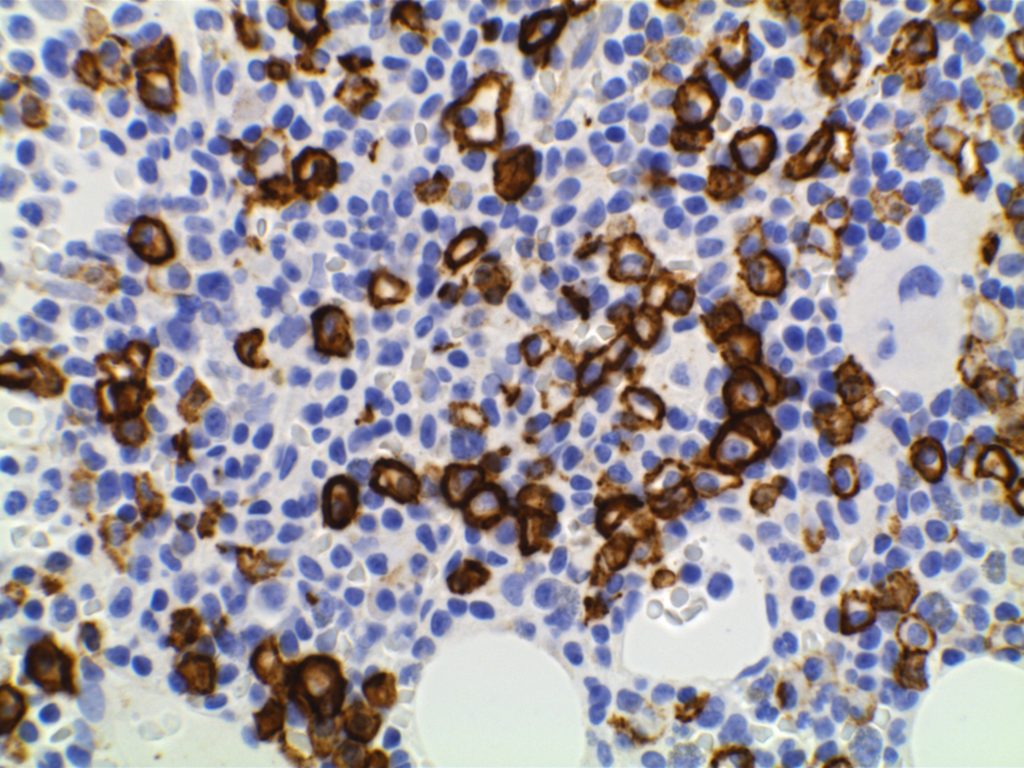
Immunophenotype
|
Marker
|
Comment
|
|
Negative
|
|
|
Negative (~10% +)
|
|
|
Negative
|
|
|
Positive
|
|
|
Negative (rare atypical cases +)
|
|
|
Positive (best marker). Marks from Mature B-Cells through Plasma Cell differentiation.
|
|
|
Often Positive
|
|
|
Positive
|
|
|
Negative
|
|
|
Positive
|
Morphology
The bone marrow can have a varying degree of involvement (usually interstitial to nodular) by tumor cells showing a range from small lymphocytes to plasmacytoid lymphocytes to plasma cells. Mast cell hyperplasia is commonly present.
References
Robbins and Cotran Pathologic Basis of Disease. V Kumar, et al. 9th Edition. Elsevier Saunders. 2015. pp. 601-602.
Harmon CM, Smith LB. B-cell Non-Hodgkin Lymphomas with Plasmacytic Differentiation. Surg Pathol Clin. 2016;9: 11–28. doi:10.1016/j.path.2015.09.007
WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. SH Swerdlow, et al. International Agency for Research on Cancer. Lyon, 2008. p. 194-195
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127: 2375–2390. doi:10.1182/blood-2016-01-643569
Martinez-Lopez A, Curiel-Olmo S, Mollejo M, Cereceda L, Martinez N, Montes-Moreno S, et al. MYD88 (L265P) somatic mutation in marginal zone B-cell lymphoma. Am J Surg Pathol. 2015;39: 644–651. doi:10.1097/PAS.0000000000000411
Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012;367: 826–833. doi:10.1056/NEJMoa1200710
Bone Marrow IHC. Torlakovic, EE, et. al. American Society for Clinical Pathology Pathology Press © 2009. pp. 27.
Hematopathology. [edited by] Jaffe, ES. 1st. ed. Elsevier, Inc. © 2011. pp.194-195.